Sunday, December 27, 2009
E.on Energy Debate, London Dec3, 2009
I had intended to embed the video, but the code is not working, at least on this laptop. In the meantime, here is a link to the E.on Energy Debate (really more a Question Time), which this blogger attended. He was in fact privileged to be invited to ask the first question - see right hand margin re "How sensible is a one-size-fits-all solution to global energy policy?
Update: I have just discovered to my disgust that the Telegraph has deleted virtually* ALLof the E.on-sponsored content. And I mean everything - every one of the 10 weekly articles from Andrew Charlesworth, the staff journalist, and every one of the 3, sometimes 4 "reader" blogs that accompanied them, mine included, together with the comments they attracted.
This is shabby behaviour by any standard, Telegraph, and the fact that you now have a sponsored series going with Shell is no doubt the reason for wiping the record. but invited comment too? Yes, Kate Day appeared on My Telegraph, inviting folk to sign up for the 10 week series. I shall say no more on the matter just now. Words fail me.
Here is a C&P of the blogs I contributed over a 10 week period. I'd have saved the comments too if I had had a crystal ball.
* Googling has turned up just one archaeological specimen, a video clip from the Energy Debate with Panel introductions. Without this, one would hardly know there had ever been a sponsored series with E.om, such is the efficiency of the Kremlin-style airbrushing of history.
Week 1 post: Pumped up about water-power
Who can doubt that the future lies with renewable energy, and that we Brits are blessed with the stuff – existing or yet-to-be-realized.
First, there are those wind turbines – not the stuff of Wordsworthian rapture I grant you - but they are increasingly being sited offshore.
Then there’s solar energy, with a choice of two panels for your roof – the older thermal, or the state-of-the-art PV panels that can feed the electricity you don’t use into the National Grid.
And there’s wave power – which is a kind of secondhand solar power, recalling that weather and wind are due to unequal heating of the Earth’s surface.
And there’s even the dear old man in the moon, not wishing to be outshone by his flashy big brother, who contributes the prospect of tidal power. Just wait until we have a hydroelectric barrage across the Severn Estuary, supplying maybe as much as 10 per cent (?) of our power supply. (Yes, there are downsides, needless to say, to any big scheme, in terms of amenity, effect on wildlife, capital cost, the carbon-footprint of setting up etc. But let’s stick with the broad brush today.)
The problem with most of the renewable schemes is that the end-product – electricity, that energy-carrier par excellence – is generated at scattered locations across the country, supply may be intermittent, or supply may not match demand around the clock or calendar.
Is there a solution? Yes, there probably is, though it’s not always a panacea. One is talking about big money upfront, and, more to the point, big commitment.
But unless or until fusion power becomes a reality – which may take decades, centuries even – then there is no Plan B, assuming one is not a unbudgeable climate change denialist who thinks the world's scientists in their droves have abandoned all reason in condemning those fossil fuels.
So what is the solution? Simply go to the wiki page on Pumped Hydroelectric Storage, and it’s all there.
Britain already has 4 PHS stations – two in Scotland, two in Wales, and now needs a lot more in different shapes and sizes.
The principle is simple. One has two bodies of water – a lower and upper level. When there’s a surplus of electrical power, say from wind farms during the night, water is pumped from the lower to the upper level. When there’s extra demand, and the conventional stations are struggling to cope, water runs back through turbines, generating electricity.
It’s the closest one can get to “storing” electricity as the potential energy of a head of water. What’s more, the efficiency is surprisingly high – 80 per cent or more they claim in a well-designed system.
Do read the article, to see the new and imaginative ways of developing the principle. The Japanese have used the sea on Okinawa as one of the two levels, the other being a reservoir at the top of the headland.
The Danes have a plan that does not even need two levels – the water is simply pumped into a giant bladder which gradually plumps up, creating its own head. Sand is laid on top to get extra oomph.
My favourite is the salt-mine idea. We have lots of worked-out salt-mines in Cheshire and elsewhere. You pump water down into the old-workings, and site your upper reservoir on the surface. Yes, the water becomes brine, so all the equipment has to be corrosion-resistant. But there’s an upside too: once the water becomes saturated brine, it’s 20 per cent heavier than pure water, so becomes a more efficient energy-transfer medium.
What is it they say – where there’s a will, there’s a way!
Week 2 post: The Cost of Wind Power
So, it’s those wind–turbines that take centre-stage today. Practical solution or wooly-minded gesture politics? Cost-effective stop-gap measure or ruinously-expensive irrelevance? A logical and rational choice - or an expression of a closet-aversion to nuclear power?
There’s a lot more that one could say, especially in the light of:
(a) our current economic situation
See especially the mind-boggling cost (£100 billion over 10 years) of even a fairly modest expansion of wind–energy, supplying just 20% maximum of our requirements (that’s on a good day).
(b) the groundswell of anger and contempt being shown – at least on websites – against the projected costs of this Government’s decarbonisation programme. See, for example, Ceri Radford’s article today, entitled: “Global warming debate is too hot to handle” .
and, finally
(c) the strange focus on just one renewable energy source, with little more than lip-service to the others ( wave and tidal power, geothermal energy, solar panels, thermal panels, biomass, nuclear energy, home insulation, heat pumps etc).
All is not lost. If like me you are a tad “green“ in your outlook, worryingly so perhaps to fellow-Telegraph readers, you may feel one should be “ doing one’s bit”. If so, then consult the Government’s website on grants for renewable energy.
Don’t be put off by the Brave New Client State listings as to who qualifies. If you’re self-reliant, ie not on benefits, then it’s just freebie energy-saving light bulbs and subsidized gas central heating that are denied you.
Look closely and you’ll see there are grants for everyone, at least for a limited period, regardless of income, for anyone wishing to go green, even for your very own wind-turbine or hydroelectric scheme.
Whether you have any spare cash after paying for the next generation of windmills (and Government IOUs) about which you were unconsulted remains to be seen!
Read James Lovelock on the subject of wind-power. He doesn’t mince his words!
Week 3 post: What about our economic survival?
Almost every headline these days with “energy” in the title adds to my despair. This last week we’ve been told there’s to be a levy on our energy bills to pay for “clean carbon technology”.
Clean carbon? Wot, finding places we can squirrel away CO2 for a few decades (hopefully) in order to meet carbon-undertakings entered into lightly? (No country with a trillion pound national debt, rising by the minute, should be making such commitments).
Then we read that there’s to be a catch-up programme on nuclear power stations, which are back in fashion, but no subsidy. Why one for coal, but not for nuclear? Where’s the logic in that?
Then we read that a £100 billion is to be spent in the next ten years on wind turbines. So how come Spain is able to generate 50% of its energy, admittedly on a good day, with turbines costing less than a billion?
Why are we, a strapped-nation, attempting to find these staggering sums of money – better spent on paying down debt – when other nations can do it at a fraction of the cost?
Yes, we know about the extra costs of siting turbines offshore. But why have rotor costs doubled in two years? And why are we still importing ours? Why aren’t we making them ourselves, as Rolls-Royce pointedly asked yesterday? And if it’s a mixed bag of energy we seek, then why no Govt campaign to cut our domestic bills, by installing heat pumps, thermal or PV panels etc?
Why so little publicity to the available grants for renewable energy? Is it because they are unofficial, offered by the industry, despite appearing on a Govt website?
Yes, let’s have that Severn barrage. It should have been started in 1973, with the first oil crisis. Which leads me on to national strategic considerations, to do mainly with oil – and COAL.
North Sea Oil is running out – natural gas as well. Where’s the National Plan to cope with rising import bills, with the renewed threat of blackmail by dem furreners Where’s the joined-up thinking?
Have a look at the Forbes website: It describes how apartheid S. Africa, and before it Nazi Germany, dealt with the threat of oil blackmail. Not the nicest of historical precedents I grant you, but let’s focus on the science.
Converting coal to oil (CTO*) is a ready-made technology which supplies S. Africa with 40% of its oil. Why aren’t we, a nation with several centuries-worth of coal under our feet, not doing the same?
The present stumbling block is, needless to say, our new national obsession – the carbon footprint. A CTO station produces two and half times the CO2 of an oil refinery, we’re told.
My reaction is to say, so what? The aim is to reduce our NATIONAL carbon footprint – not to impose an economic strait jacket on every local or promising new development with strategic as well as economic considerations.
We as a nation urgently need to maintain our AAA credit status if we are to remain solvent. What better signal than to announce that it’s our aim to become largely self-sufficient in energy, especially in oil and gas that appear on the balance of payments figures. Coal can give us coal gas (50% hydrogen!), oil, coke (for our steel industry) and many other goodies besides.
If we are going down the road of burying CO2, then let’s start with CTO if one has to. But I’d say, cut development and running costs by venting CO2 into the atmosphere, but offset with a programme of subsidized home improvements to reduce CO2 elsewhere.
We are fighting for our economic survival goddamit! Aka CTL (coal-to-liquids).
P.S. The writer has no personal financial stake in any of the above, except as the recipient of modest pensions dependent on future stock market performance.
Week 4 post: Blame the Government and the energy suppliers:
The Government and energy suppliers are getting it all wrong. They are attempting to market future power supplies as if they were a desirable and seductive consumer product - a new car or a plasma TV.
If one’s talking of a shiny new fashion accessory, then a group of investors finds the start-up capital, maybe going to the money markets.
The product is then priced so as to make a return on that possibly risky investment, and also gradually (very gradually) to recoup the capital investment .
But one should not be applying the same principle to a boring old utility company (sorry, Eon, but when did you last see an undignified scramble on Monopoly for Waterworks and Electricity?).
There is no new product – gas, electricity etc. It’s the same old product, but with the prospect of ever -increasing bills. Yes, that’s the difference between launching a new saloon car, and generating more bog-standard electricity, if you’ll pardon the expression – RISK – of which there is much, much less in the utilities industries– and the business model should reflect that.
It’s probably not a good idea to use a blog to float a brainwave – least of all one that has only just entered one’s head, with a theme that may not be Mozart to the sponsor’s ears. But here goes.
The consumer must not be taken for granted. The consumer needs a carrot as well as a stick.
Here’s what I suggest. The Government announces an additional VOLUNTARY levy on fuel bills calculated to cover the capital cost of new nuclear power stations, wind farms etc.
The first million who sign up will get a 10 per cent discount, in perpetuity, on their bills. The next million that sign up will get a 9 per cent discount. The more numerate readers can probably detect a pattern here - one that should allow them to work it out for successive tranches… ;-)
So stop taking us energy consumers for granted. Industry and government need our goodwill and cooperation to meet its carbon targets, and to achieve greater economies in our homes.
So kindly cut us in please on some of the investment returns, given we are expected to put up some or all of the risk capital. Failure to do so will simply serve to generate a new wave of eco-cynicism – one that will destroy the incentive for individual initiative.
Or is no place seen for the latter in our brave new post-privatization world of State-supervised near-Monopoly?
Week 5a post: Carbon Capture and Storage
So it’s coal that will plug the gap in our energy supply, is it? That’s provided we can bury the evidence (CO2).
And that’s the long and the short of it: we sign up to agreements one day, promising hand-on-heart that we will honour our CO2 obligations – and then, and only then, decide if we have the technology.
And who’s to provide the pot of gold for R&D? Yes, you guessed correctly. There’s to be a special levy on consumers, with no guarantee the technology will work. Even if successful in a narrow technical sense, would it not be replacing one putative hazard (atmospheric) with another one (geological) that could haunt future generations?
First, let’s be clear about what is proposed. It sounds simple and straightforward if you say it quickly: CO2 will be pumped underground where it will remain for thousands of years, or so we are assured. See this BBC feature on CCS
But when you look at the detail, the technology proposed is not just optimistic – e.g. finding the right geological strata capable of concealing billions of tonnes of CO2 under the biospheric carpet, out of sight, out of mind (?), it’s positively hair-raising.
Firstly, it’s not CO2 GAS that’s being pumped underground, but LIQUID CO2 – a refrigerant by any other name – needing huge pressures (70 times atmospheric!) to make and keep it liquid.
So where’s all this volatile refrigerant to be stored, where it can be kept under pressure to stop it turning back to a gas?
Answer: suitable spots in the Earth’s crust at least 800 metres deep. You see, there has to be the weight of millions of tons of overlying rock on top of to prevent it vaporizing back to gas. So the idea is basically to do what’s done in oil drilling, but in reverse.
Contrary to common belief, the crude oil down there is not in lakes. It’s dispersed throughout the pores of solid rock. It’s the pressure of overlying rock that brings it up to the surface. So the idea is to find porous rock down there, and then inject liquid CO2 under pressure.
Does this not strike you as a heroic, possibly foolhardy thing to do? How is the lid to be kept on this potentially self-propellant liquid CO2? By means of a “geological cap”, we are told. See that BBC website
And it’s warm down there too, is it not? When I read all this, I’m reminded of a rather naughty thing I used to do as a child with our pressure cooker. If left to cook the potatoes, then instead of cooling the cooker under running water, as prescribed, I’d get a fork, and simply lift off the collection of weights that were the safety valve.
The jet of steam was spectacular, hitting the ceiling, and lasting for half minute or more! Embryonic scientists learn to live dangerously!
I cannot help but recall that steam-spectacular when reading that liquefied CO2, under high pressure, is to be stored underground, relying upon a “geological cap” to keep the lid on things. Yeah, right. And what if CO2 did suddenly leak out – creating a dense carpet of suffocating gas?
And we’re picking up the tab to turn a boffin’s wild-eyed dream of “safely locked away” CO2 into reality… Dream or the stuff of nightmares? Is there a sane geologist in the house?
Week 5b post: Doubts about CCS
Still on “carbon -capture and storage” (CCS) – all £10 billion pounds worth upfront in the form of fuel levies (says he with a deep sigh): the more I think about it, the less I like it.
To recap: the plan is to dispose of CO2 gas from power stations by piping the gas (well, liquid actually) underground. The preferred geology, we are told, are porous rock layers deep underground, overlaid with a “geological cap”. It’s the same geology, we are assured, that kept crude oil trapped under ground for millions of years until the day our oil drills penetrated.. If it worked for crude oil, then - beware the Cheshire cat grin - why not for liquefied CO2? More about Cheshire later…
Hold on a minute. Did anyone else apart from me do O-Level Chemistry? Carbon dioxide reacts with water to make carbonic acid (H2CO3). And water is everywhere – especially deep underground.
Carbonic acid gradually attacks and dissolves limestone (calcium carbonate, CaCO3). And guess what: limestone is the preferred rock into which to inject CO2. It’s like a sponge we are told (“porous structure”).
So the limestone would gradually get eaten away and hollowed out (the timescale is anybody’s guess). Caverns and chambers will form. But that can’t go on forever. Sooner or later the roof will collapse (overlying pressure). Think old salt workings, Cheshire, surface subsidence, bye bye your home-sweet-home. “Dear insurers, I am writing this from a temporary address…”
For a dramatic (well OK, slightly over-dramatic) representation, see a recent post of mine on My Telegraph (scroll down to last comment).
Time scale? Who can say? But high local concentrations of CO2 and subterranean warmth (geothermal energy) would both favour rapid solution of limestone. So how good would that “geological cap” be if keeps periodically collapsing into underground caverns?
And what happens to dissolved limestone? Well, it changes to soluble calcium bicarbonate (aka hydrogen carbonate, Ca(HCO3)2). But calcium bicarbonate is chemically unstable: it reverts back to calcium carbonate (“chalk”) and CO2. It’s why pipes and kettles fur up in hard water districts, and what creates stalactites and stalagmites. Reforming of chalk is the least problem: MORE IMPORTANTLY, the CO2 gas would inevitably be re-released at a later date, seeping back gradually into the atmosphere or the oceans. Ever heard the term “futile cycle”?
In fact, there is another more fundamental objection to the entire strategy of burying future emissions of CO2. According to some theoreticians, CO2 has already produced most of its greenhouse gas/global warming effect. See the this article.
cIf global warming is still in progress – and only the statistical illiterati can think otherwise – then it’s not due to additional CO2. What is responsible? Methane? Soot? Positive feedback effects (eg melting Greenland ice exposes rock with decreased albedo, greater absorption of solar radiation, still more infrared, more warming?
The logic of that case, if true, is that prevention of further CO2 increase is not enough. Sooner or later we must engineer a DECREASE in CO2.
How can that be done? Conversion of biomass to inert charcoal (“biochar”) has been touted as a radical solution. The carbon must on no account be burned, but could still have uses, eg as a soil conditioner. (Such as shame to waste a valuable fuel, but it’s not wasting carbon – fossil carbon – that got us into this trouble).
Pyrolysis of wood etc to biochar is at best energy-neutral, and probably has an energy cost. It would have to be supplied from renewable energy to make any sense. That would mean less energy for power generation.
Without wishing to sound alarmist, we are in a real fix, folks – make no mistake about it.
Week 6 post: The problem with gas
So, the main focus this week has switched to gas. Hmm. What can one usefully say about gas? Well, starting in the late 60s/early 70s, we used to have lots of it from the North Sea. How long ago that all seems now – like the man coming to convert our gas cookers.
Well, we’re now told that natural gas is running out. I never did trust those finite resources! We are now forced to import more and more –but in specially converted tankers - as liquefied natural gas . Dodgy - very dodgy. Enough said.
Are there substitutes for methane that we could make ourselves, to cut our imports bill?
Probably not. Sure, we used to pipe coal gas to people’s homes. But the paramedics and hospitals hated it – need I say more - and gasworks were smelly.
What about a renewed role for coal – not a popular line to take right now, least of all with those gathering right now in Copenhagen, looking to set targets on carbon emissions? (OK, so I’m straying off gas).
Personally, I don’t question the need to drastically cut greenhouse gas emissions. There will have to be a greater role for nuclear power, for renewable energy, with natural gas being a bridge. But why consign coal to oblivion on account of its high carbon footprint? Does every single process have to tick every green box?
You see, there’s coal-to-oil technology, currently used in S.Africa to replace 40% of oil imports.
The coal is gasified with injections of oxygen and steam, to make ‘syngas’ , a mixture of carbon monoxide and hydrogen . One could burn syngas – for a power station - but that would be squandering a big opportunity.
Why? Because the mixture can be passed over heated catalysts to effect the Fischer-Tropsch process . The result, to a jaded chemist, is pure magic. The mixture transforms to a range of hydrocarbons, general formula Cn H2n+2 where n starts at 1 (plain old methane CH4) but can be much much higher. When n is 8, one is producing octanes, C8H18, which are volatile liquids, ideal for use as synthetic petrol. Planes have been flown on synthetic coal-derived petrol!
But as indicated – there’s a fly in the ointment. The process creates a larger carbon footprint than does conventional refining of crude oil. That’s because it generates some CO2 as a waste product. OK, so one could sequester it underground – using that somewhat dubious CCS - but it adds considerably to the costs - some 25 to 30%. That’s quite apart from any environmental concerns one may have about creating subterranean pressure cookers full of liquefied CO2 under the Earth’s crust.
Well, I’ve strayed from gas, onto oil – albeit synthetic oil - derived from coal.
Yes, I do see a revived role for coal.
Here’s a possible scenario - one that could probably be adopted whatever targets are set at Copenhagen. We would buy in surplus nuclear- generated electricity from France as and when available (it’ll take years to build more of our own power stations). Instead of paying in euros - with a now unfavourable exchange rate- we’d enter a barter deal, swapping it for our coal-derived synthetic oil.
The carbon footprint of French nuclear electricity (very low) and coal-derived oil (high) would average out at something intermediate, possibly comparable to natural gas. So let’s keep coal in the equation, but turn it into vehicle fuel, instead of burning it all directly to CO2 in power stations, with all those expensive and questionable CCS add-ons. Let’s box clever. We’re going to need petrol for vehicles for a while to come, so let’s start making our own - and trading it.
Week 7 post: Going nuclear
The focus this week is on nuclear power. One’s first reaction is to say: “Please – anything but nuclear power!”
Just a quick internet search was sufficient to reinforce all the old misgivings. Why do nuclear power stations have so limited a lifetime – a few decades as most, then requiring horrendously expensive and hazardous disposal of nuclear wastes?
Up popped this return on the decommissioning of nuclear power plants, telling me more than I really wanted to know.
In answer to my question: because of neutron irradiation of the structure, which gradually renders it unsafe (let’s not go into details, except to say that neutrons are like miniature bullets). But the practicalities are disturbing - nay hair-raising, like the mention of having to leave the core intact for a few decades while the worst of the radiation subsides. All my instincts say NO to nuclear power.
But then the practical scientist inside me takes over. Let’s face it, there are remarkably – and depressingly – few options where energy is concerned.
They can be divided into 4 categories: Chemical, Biological, Classical Physical, Nuclear.
Let’s take Chemical first. There are 92 chemical elements in nature, but only 2 of them, free or in chemical combination with other elements, are suitable as fuels, namely hydrogen and carbon. That’s because they react with oxygen to release energy, producing relatively innocuous end products - water and CO2. Innocuous that is from a personal health view. The health of the planet is a different matter (CO2).
Hydrogen has been touted as the fuel of the future. It’s not, and never will be. Why? Because it does not occur free in nature. Obtaining it from water (by electrolysis or reaction with carbon) or from natural gas requires roughly as much energy input as the output when burned.
Hydrogen is not a real fuel – it’s better described as an energy carrier – similar to electricity. Forget hydrogen as a solution to our energy crisis.
Carbon – whether as coal, natural gas etc. is now the no no. So that’s the chemical possibilities exhausted.
Biological power? This means trapping the energy of sunlight with photosynthesis to make biofuels, or methane by fermentation, or charcoal from biomass and then burning the product.
CO2 in this instance is not a no no, because unlike fossil fuel CO2 one is only putting back into the atmosphere what was taken out. Ne would think that biological-energy (really solar) should be a panacea, but it’s not. There are too many associated problems of cost – both economic and environmental.
Classical Physics? Here I refer to renewable energy: wind, hydroelectric, wave and tidal, solar panels, heat pumps, geothermal etc which all depend on converting one kind of physical energy to another, which is usually electricity or heat.
But again – while they can be developed and expanded – they are no panacea. They simply cannot meet those three criteria simultaneously – affordable, reliable, low carbon.
That leaves us with nuclear power. Given there are still abundant reserves of uranium, that it’s not in the hands of a few suppliers, that nuclear power stations are low-carbon (though probably not as low as claimed) and that they operate round-the-clock, then there’s no escaping the conclusion that it is nuclear power that will have to fill the energy gap. That’s until fusion power becomes reality – which may take decades or longer.
It’s a bitter experience for those familiar with the history of nuclear power in Britain – e.g. the decision to develop our own AGR and Magnox technologies, that have not proved as safe and or as economical as the PWR reactors developed in the USA, France etc. Then there was the Windscale incident, and then Chernobyl that all but killed off the UK industry.
I guess we have no choice now but to re-habilitate nuclear power in the UK – and fast – unless we want the lights going out!
Week 8a: Time to reinvent oneself
One’s first thought is unprintable. Does one really want to be bothered with a whole lot of new paraphernalia in and around the house? Does one really want to become an energy-efficient obsessive? Will one suffer terrible pangs of conscience for leaving lights burning? Or luxuriating under a hot shower for another minute or two. Will one feel like an eco-Neanderthal to be the last house in the street without solar panels?
The immediate answer to all these is a forceful NO WAY. But then one thinks to oneself : hold on a min, one’s a baby-boomer who’s probably enjoyed halcyon years the like of which will never be repeated. OK, so it didn’t always seem like clover, like that time interest rates hit 15 per cent, and I was seriously thinking of erecting a tent in my first new house to reduce heating bills, and pinned sheets of polythene across all the windows. But there were those generous student grants, plus the mortgage interest relief and other subsidies that sound bizarre now.
Then one remembers the austerity years – the 50s – before Super Mac told us we’d never had it so good. One of the daily rituals was making up the coal fire. One needed a stock of newspaper – annoying because the chippy would pay you 6d for a decent-sized stack. Then there was the kindling – which cost 6d – or even firelighters if you were posh, or a gas-poker if you were the Jones, and finally the coal. Who can forget the heart-in-mouth experience of waiting to see if the fire “took” before one had exhausted one’s meagre stock of material?
So this veteran of both softer and harder times says – time to reinvent oneself, Sunny Jim, and become an eco-obsessive, one for whom energy economy becomes part of one’s daily routine.
So where would I start? A well-insulated hot water tank, obviously. It can pay for itself in weeks, they say. Then loft insulation. Double the thickness, even if it means ditching all those seems-a-shame-to-throw-them-away items. Ditch – that’s an order ! (trust me, you’ll feel much better when the deed is done).
Then cavity wall insulation – and get any grants that are going.
Don’t stop with the cavity, though. Put up pine-panelling inside, with an air gap. Even more insulation – and you’ve sequestered some carbon into the bargain. Let a Scottish or Scandinavian forest make some more wood, taking up more CO2. Maybe laminate flooring too, with a good quality insulating underlay.
Then install double-glazing if one hasn’t already done so. Cost-effectiveness? Let’s keep that for comments.
Then consider a replacement gas boiler, preferably a double-condenser. Cost effectiveness? As above.
Then one thinks about the big ticket items. Hopefully there will be new grants and subsidies shortly – provided the whole thing doesn’t get bogged down in party politics.
My first preference would be for photovoltaic (PV panels) with a feed to the National Grid, allowing one to sell any surplus. But without a grant to defray the big bill for all that photoelectric silicon, I’d probably settle for good old-fashioned thermal panels, the sort with an intermediate heat-exchanging fluid.
Heat pumps? If one has a big plot for buried heat-exchangers, then yes. Otherwise it’s probably not practical or cost-effective.
Forty words left. Must make each of them count. Damn – have used 14 already. Nope, don’t want a wind turbine. Oh yes, I know. It’s been at the back of my mind for ages. Turn down the thermostats, wear more pullovers (not hairshirts) , and watch the blight of the baby-boomer generation slip away - inch-by-inch - with an increased basal metabolic rate. The fat of the land… (ed – best stop there Colin, you’re over your word limit anyway).
Week 8b post: Colin on COP15
Well now, would you believe it? I’ve been asked by that nice Maya at Blog Central to provide an update. Carte blanche too!
They'll be asking me to be caretaker on the whole front page at this rate, while they nip out to get their Christmas shopping done.
What to talk about? Copenhagen? Oh my! What a disaster! Of course, some of us saw it coming. Here's the question I put to the panel at the E.on Energy Question Time, London, Dec 3: "Re Copenhagen: how sensible is a one-size-fits-all policy on cutting global emissions, given there are other environmental considerations, eg oil stock conservation?"
Supplementary: Might one not envision a smart-deal in which Britain concentrates, say, on converting her abundant coal to petrol, thus conserving oil for organic synthesis (polymers etc) while countries like China and India would concentrate on cutting their CO2 emissions?
Personally, I thought it madness to imagine that a few magic fountain pens could be waved over documents to secure a worldwide agreement.
Whoops. There's my own Xmas shopping still to to complete, Here, with apologies, is a lazy way of concluding, with a copy‘n'paste of what I said yesterday on My Telegraph re Copenhagen, which brought the sky tumbling down on my head (strange place, MyT ;-):
"It's interesting to compare the successful Montreal protocol, phasing out CFCs, with the now failed attempts to reproduce them with Kyoto and Copenhagen. Turning off the CFC tap was easy, because it only required legislation, applied to manufacturers.
Turning off, or at rate down, the anthropogenic greenhouse gas tap is infinitely more difficult, because legislation cannot be applied to individuals - thus the attempt to substitute the quasi-legislative carbon trading at the industrial level, with all the opportunities it offers the opportunists. The whole shebang has been mishandled and misconceived from the word go. Fossil fuels have played a vital role in developing the planet.
One cannot demonise them at the stroke of a pen - or the broadcasting of a few politician's soundbites - and expect the entire world to reinvent itself - especially if that involves re-inventing the windmill. Back to the drawing board, you world so-called leaders. This time, try leading instead of imagining you could solve this one by waving a few magic fountain pens.”
http://my.telegraph.co.uk/china_jo/blog/2009/12/20/for_chl
Comments welcome, natch
Week 9 post: Response to Andrew Charlesworth
Response to Andrew Charlesworth’s “On the road to Decarbonisation): Well, Andrew, the ideas that you outline sound great in theory, and one can see why the energy suppliers are keen on them. First, we trade in our petrol/diesel vehicles for electric ones. Then we recharge them at night, using renewable energy from wind turbines etc. That then helps to balance the load on the power supply – we would be recharging when demand for cookers, washing machines etc is low, but the blades are still a turning'o. And we, as car purchasers, would be the ones who would have to cough up for all those expensive storage batteries. Ingenious!
Yes it fits the bill nicely if you are an energy supplier, caught painfully on the over-prolific horns of a trilemma, looking for affordability, reliability and low-carbon simultaneously.
But once it ceases to be purely about generation, and involves the consumer as end-user – then yet another horn sprouts forth - that of convenience. Yes, CONVENIENCE. We are now into quadrilemma territory, with more protests from my spellcheck .
Let’s be clear about one thing. Electric cars do have their uses. They are ideal for congested cities. Why? Because they are zero emissions. That’s not just zero CO2, but zero CO, SO2, NOx and all without those expensive catalytic converters. They are fine as a runabout with low predictable mileages. But they are NOT suitable as general purpose family cars. The reasons are obvious. Once the battery runs flat, it takes hours to recharge. What’s more, one would probably incur a cost penalty for doing that during the day.
Cars play too vital a role in our everyday lives to have one’s convenience curtailed in this fashion. And supposing one got an emergency call in the night, and the car was only half-charged?
Whilst I accept the need to decarbonise where possible, we should be realistic and accept there will always be a role for some CO2-emitting operations. The trick is to replace fossil fuel. One can do that with biofuels, although they are controversial, removing arable land from food production.
There are alternatives. Synthetic petrol can be made from coal via so-called syngas– admittedly with a large carbon footprint. But the same technology could be applied to wood charcoal and other sources of “biochar”, using managed forestry, waste processing etc. The power needs of the process (carbon to syngas to liquid hydrocarbons) can also be met using renewable energy to minimize the carbon footprint.
There’s an intermediate solution – using hydrogen instead of electricity as the energy carrier. But it’s no panacea. It’s not a true fuel – needing energy to produce from water, methane etc – and also having problems of low-range, safety considerations etc.
My interim solution would be based on biochar. Use half of it for synthetic petrol, and the other half as a soil conditioner, applied to marginal land to raise its biomass output.
It’s easy in a fit of crusading zeal to demonise fossil fuels and, from there, all CO2 – including recycling biomass CO2. That is illogical. Here’s a comment from “OntheDot” that appeared on my Week 8a post (“Views on the Climate Change Conference at Copenhagen) that I would recommend to world leaders while licking their post-Cop15 wounds, and hatching their amended plans for Mexico:
“We use energy in three different ways:
1 Electricity; the most useful producer of this would be Nuclear energy.
2 Heating; the most efficient method is gas.
3 Transportation; The only reliable method is liquid hydrocarbons.
Until politicians realise this can we begin to plan accordingly. At the moment we use our energy in a haphazard manner to the detriment of all.”
Thanks OntheDot. Couldn’t have put it better myself.
Week 10 (final post): Diversity and Economy are the Key
Two clear messages have emerged during the ten weeks this series of sponsored features has been running. They can summed up in two words - DIVERSITY (of energy supply) coupled with ECONOMY (at the point of use). The drivers are also two-fold – the need to REDUCE EMISSIONS of greenhouse gases, primarily CO2, and the UK’s INCREASING RELIANCE on imported fuel, notably crude oil, natural gas, and not forgetting uranium for the next generation of nuclear power stations.
Personally, I think it’s a shame we have to stop the discussion now. Why? Because there is, to my way of thinking, a largely unexplored conflict between reducing emissions on the one hand, and watching the national import bill rise on the other. Whether one views decarbonisation as an act of self-interest or one of altruism – or even a forlorn and futile act of gesture politics – given the outcome of Cop15- it’s coming at a time when Britain is ceasing to be self-sufficient in fuel. So we face all the horrendous costs of decarbonisation, estimated at £20 billion a year for the next 10 years, while at the same time we are obliged now to buy fuel with the prospect of volatile spot prices. So we volunteer to be saints on the one hand, while risk being treated as sinners with the next crisis affecting crude oil or gas supplies.
I’ve already hinted in earlier posts to some possible approaches that may help to square the circle. The first is to abandon the absurd one-size-fits-all approach adopted by the major Western nations at Cop15. The major sticking point there was China, along with India and the other rapidly emerging economies. They were offered a stick with no carrot (unless one regards a lessening of the immediate threat of catastrophic climate change as sufficient of an inducement to accept the West’s and UN’s proposals).
If China is to be persuaded to cut its massive discharges of largely coal-derived CO2, it has to be offered a more tangible quid pro quo. What might that be? Security of crude oil supplies – at a price that can be afforded - might just swing it. That’s where other coal-rich nations like Britain can contribute – by developing the coal-to-oil technology that has been so successfully developed by S.Africa, and maybe extending it to biochar-to-oil as well, thereby reducing the net carbon footprint.
My own feeling is that enlightened self-interest is always a better bet where international relations are concerned. Britain needs to reduce its reliance on imported oil, and can do that using its abundant reserves of coal. If we produced a surplus of oil, some could be bartered in exchange for French nuclear electricity between now and 2020 while we catch-up on nuclear power. The carrot? Convincing China that a reduced demand from Britain and other coal-to-oil nations will help to prevent repetitions of the oil price hike that occurred in 2006 – when prices trebled over an 18 month period.
Gettting China – now reckoned to be the second largest economy in the world – to play ball will not be easy – and there may be solutions more realistic or imaginative than the ones proposed here. What’s for certain is that unilateral – or even multilateral agreements on carbon emissions - will be futile unless China, and then India, Brazil, Russia etc are brought on board. One hopes the lessons learned at Cop15 will not be repeated next time around in Mexico. The clock is ticking where global temperature rise is concerned.
Saturday, November 7, 2009
The origins of life, nucleic acids, purines, pyrimidines and extra-terrestrial cyanide
Newsflash: Monday 10th October, 2016
Have just come across this item in the Mail.
http://www.dailymail.co.uk/sciencetech/article-3830773/The-secret-ingredient-life-Earth-THICKENER-kick-started-evolution-living-creatures.html
I strongly recommend it, especially as there are faint echoes of the thinking in this posting of mine from way back in 2009.
Start of original posting:
An opportunity has just presented itself to share with my fellow earthlings an idea that's been fermenting in this senescent brain for some time. It concerns the building blocks of DNA and RNA - the purine and pyrimidine bases. These are the flat stackeable molecules that make up the base pairs in double-stranded DNA - Watson and Crick's double helix- and thence the triplet genetic code.
Left: pyrimidines and purines. Right: a pyrimidine nucleotide showing the intermediate ribose sugar between a pyrimidine base and phosphate
Why were these particular molecules selected from the primeval soup? Could other molecules have served in their place? Did adenine and guanine (the purines) and cytosine, thymine and uracil (the pyrimidines) just happen to be "lying around", so to speak, in some primeval rock pool, or forming in the vicinity of a hydrothermal vent?
I tried tackling it first from a physicochemical standpoint. What conditions would be needed for any kind of spontaneous chemistry to occur in a puddle say on Earth? The classic Miller-Urey experiments you may recall subjected the assumed constituents of Earth's proto-atmosphere to electric discharges, in an attempt to generate the precursors of proteins and nucleic acids (amino acids, purines and pyrimidines). Glycine - the simplest of the amino acids- was formed, but that was hardly a chemical cornucopia.
The Miller-Urey apparatus. The test mixture of water, methane, ammonia, hydrogen and carbon monoxide was intended to replicate the then-imagined reducing atmosphere. Current thinking is that the main carbon component was CO2 rather than methane CH4. Interestingly, substituting CO2 for methane gives the same range of amino acid and other products, provided that oxygen is excluded.
See wiki for more details
See wiki for more details
One of the chief problems would have been intense uv radiation from space - without oxygen, there would have been no ozone "sun-screen". So maybe the first requirement was to generate sun-screen agents in situ. Purines and pyrimidines all absorb strongly in the ultraviolet:
From left: adenine ( as the nucleotide AMP ); uracil (as UMP); cytosine (as CMP); guanine (as GMP)
So if they formed by accident a kind of proto-Darwinian natural selection would operate. Further molecules could then evolve, protected by the sun-screen molecules. What about the sugars - the five carbon ribose and deoxyribose? Could they have developed initially in a permissive role, simply to allow more chemistry to proceed?
If life did evolve on land, say in a rock pool receiving intermittent rain, and exposed to all weathers, then there needed to be a mechanism to protect against freezing or evaporation. Chemistry generally needs to be in solution if one is to synthesise. Well, there's a possible role for sugars. Firstly, they depress freezing point, ie have anti-freeze properties. What's more, a sugar solution tends to go syrupy when water is evaporated. It's often quite difficult to drive off the last of the water. Sugar molecules bristle with hydrogen-bonding OH groups which cling tenaciously to water.
Sun-screen agent, anti-freeze - are these the properties of purines, pyrimidines and sugars that caused them to become the building blocks of life?
What about those five chemical bases? Where did the carbon and nitrogen come from for their cyclic structures?
It was the article in the current New Scientist ("Was life founded on cyanide from space crashes?") that provided another piece of the jigsaw. It's proposed that life on earth was seeded by cyanide , -CN, in asteroids. As written it's a free radical, not a stable molecule. It would have to be HCN, or a metal cyanide, eg KCN. Now if you look at the detailed structure of purines and pyrimidines, you will see that all of them feature an alternation of -C-N- in their rings. There are some -C-C bonds as well, but the cyanide dimer cyanogen N-C-C-N could have provided that.
As soon as I started googling I struck pay dirt, with a 1978 paper describing how solutions of HCN began to form purines and pyrimidines spontaneously in the presence of alkali.
Read the New Scientist article and comments, five at the time of writing, with the suggestion that cyanide is the carbon precursor par excellence, not only for its association with nitrogen, but for being "electron-rich" (my term), given the presence of that triple bond between the two atoms, representing three shared pairs of electrons.
Standard valence formula (lower left); electronic configuration (upper right). Of the six electrons in the triple bond, 4 are available for forming new chemical bonds with additional atoms of carbon, nitrogen, hydrogen etc.
Further reading: for a critique of the Miller-Urey experiment, indeed, the entire idea that life could have evolved spontaneously, see the article by John Peet.
Postscript: it's all been thought of before: at least the cyanide to purines route.
And, to end on a light note:
Postscript: added October 8, 2016:
It's nigh on 7 years since penning this post, which still continues to receive a trickle of visitors, according to my sitemeter. Much water has flowed undr this blogger's bridge these last few years. First there's this retired science bod's explanation for the Turin Shroud - a flour/oil imprint from a live adult male (probably 13th century!) onto linen intitially, then roasted, then washed, to produce the final faint, enigmatic 'negative' image. See my specialist TS site for details.
There's also my theory for Stonehenge, and indeed most standing stones, circles and henges in the UK, namely that they were sites for "sky burial", or as I prefer to call it, AFS (avian-facilitated skeletonization). See the most recent postings on this site for the basis of this interpreation, much resting on the crude and rustic layout of what has been dubbed "Seahenge" uncovered by storms on the Norfolk coast in the late 1990s.
Labels:
cyanide,
DNA,
New Scientist,
nucleic acids,
origin of life,
purines,
pyrimidines
Friday, October 30, 2009
Talking Energy - an individual perspective
Today's the day when the Telegraph launches its "Talking Energy" feature in association with E.on, the energy supplier.
One is invited to join the debate . Four of us bloggers are each given individual "home pages" on the website: Christina O, SabinaA, the recently-arrived Robert and myself.)
Whether, in the case of E.on, one calls it a public consultation exercise or a PR campaign, does not worry this blogger unduly. Given the way that the AGW debate has been politicized and polarized, any new forum is to be welcomed if it allows the voice of reason and real science to be heard without all the personal aggro.
 So this blogger has signed up, asking that any new forum be proactively moderated. I suggested in an email that it could usefully take a leaf from New Scientist about how to moderate. (For example, the NS has no time or patience for those persistent conspiracy theorists who clutter up its comments, and a good thing too.)
So this blogger has signed up, asking that any new forum be proactively moderated. I suggested in an email that it could usefully take a leaf from New Scientist about how to moderate. (For example, the NS has no time or patience for those persistent conspiracy theorists who clutter up its comments, and a good thing too.)
"Reactive moderation", as one sees on My.Telegraph, may be cost-effective, but it degenerates quickly into unseemly dogfights, ending with entire posts and comments - good as well as bad - being deleted by the tardy moderators.
I received an electronic galley proof of the Telegraph/Eon opening gambit, scheduled for later today tomorrow, and submitted my 600 words yesterday, along with mugshot and bio' as requested. One waits with bated breath (more commonly rendered these days as "baited breath", with visions of mouse traps and Cheddar cheese*).
I'm not giving any secrets away by telling you that the central message of the exercise is that energy companies like E.on are confronted with what they call a "trilemma". (A parallel campaign on YouTube whence came my graphics is already underway with that questionable t-word neologism in prominence). The trilemma refers to so-called "conflicting" demands that future energy supplies be: 1. Green (low-carbon) 2. Affordable and 3. Reliable.
Well, one could be pedantic and say there's nothing conflicting there - they are merely three desirable criteria that need to be met simultaneously. Otherwise our lives would be burdened with trilemma, like where to live (it's got to be a nice area, affordable, handy for public transport).
Rather than nitpick, this blogger decided to focus on renewable energy, and how it might be made more reliable, given fluctuations in wind speed affecting turbine output etc. Clue: Dinorwig.
I'll let the Telegraph publish first, then wait a day or two.
Watch this space!
* Cruel Clever Cat by Geoffrey Taylor:
This little ditty first appeared in an anthology called Catscript, edited by Marie Angel. However, it was first published in 1933 in a limited edition of Geoffrey Taylor’s poems entitled A Dash of Garlic.
Additional reading E.on powers down to cut carbon and costs
Interesting to see the spotlight being put on our PCs - and rightly so, when you consider the heat that comes - not just from the laptop itself, but the charger too.
Energy-saving tip: when using one's laptop, switch off a light or other appliance that would normally be on, if it's superfluous to requirements when you are online.
Update: Thur 5th November: Here's a C&P of my first post on the E.on site. Having been up for a few days now, it would not seem a terrible breach of protocol to add it here for archival purposes:
Who can doubt that the future lies with renewable energy, and that we Brits are blessed with the stuff – existing or yet-to-be-realized.
First, there are those wind turbines – not the stuff of Wordsworthian rapture I grant you - but they are increasingly being sited offshore.
Then there’s solar energy, with a choice of two panels for your roof – the older thermal, or the state-of-the-art PV panels that can feed the electricity you don’t use into the National Grid.
And there’s wave power – which is a kind of secondhand solar power, recalling that weather and wind are due to unequal heating of the Earth’s surface.
And there’s even the dear old man in the moon, not wishing to be outshone by his flashy big brother, who contributes the prospect of tidal power. Just wait until we have a hydroelectric barrage across the Severn Estuary, supplying maybe as much as 10 per cent (?) of our power supply. (Yes, there are downsides, needless to say, to any big scheme, in terms of amenity, effect on wildlife, capital cost, the carbon-footprint of setting up etc. But let’s stick with the broad brush today.)
The problem with most of the renewable schemes is that the end-product – electricity, that energy-carrier par excellence – is generated at scattered locations across the country, supply may be intermittent, or supply may not match demand around the clock or calendar.
Is there a solution? Yes, there probably is, though it’s not always a panacea. One is talking about big money upfront, and, more to the point, big commitment.
But unless or until fusion power becomes a reality – which may take decades, centuries even – then there is no Plan B, assuming one is not a unbudgeable climate change denialist who thinks the world's scientists in their droves have abandoned all reason in condemning those fossil fuels.
So what is the solution? Simply go to the wiki page on Pumped Hydroelectric Storage, and it’s all there.
Britain already has 4 PHS stations – two in Scotland, two in Wales, and now needs a lot more in different shapes and sizes.
The principle is simple. One has two bodies of water – a lower and upper level. When there’s a surplus of electrical power, say from wind farms during the night, water is pumped from the lower to the upper level. When there’s extra demand, and the conventional stations are struggling to cope, water runs back through turbines, generating electricity.
It’s the closest one can get to “storing” electricity as the potential energy of a head of water. What’s more, the efficiency is surprisingly high – 80 per cent or more they claim in a well-designed system.
Do read the article, to see the new and imaginative ways of developing the principle. The Japanese have used the sea on Okinawa as one of the two levels, the other being a reservoir at the top of the headland.
The Danes have a plan that does not even need two levels – the water is simply pumped into a giant bladder which gradually plumps up, creating its own head. Sand is laid on top to get extra oomph.
My favourite is the salt-mine idea. We have lots of worked-out salt-mines in Cheshire and elsewhere. You pump water down into the old-workings, and site your upper reservoir on the surface. Yes, the water becomes brine, so all the equipment has to be corrosion-resistant. But there’s an upside too: once the water becomes saturated brine, it’s 20 per cent heavier than pure water, so becomes a more efficient energy-transfer medium.
What is it they say – where there’s a will, there’s a way!
One is invited to join the debate . Four of us bloggers are each given individual "home pages" on the website: Christina O, SabinaA, the recently-arrived Robert and myself.)
Whether, in the case of E.on, one calls it a public consultation exercise or a PR campaign, does not worry this blogger unduly. Given the way that the AGW debate has been politicized and polarized, any new forum is to be welcomed if it allows the voice of reason and real science to be heard without all the personal aggro.
 So this blogger has signed up, asking that any new forum be proactively moderated. I suggested in an email that it could usefully take a leaf from New Scientist about how to moderate. (For example, the NS has no time or patience for those persistent conspiracy theorists who clutter up its comments, and a good thing too.)
So this blogger has signed up, asking that any new forum be proactively moderated. I suggested in an email that it could usefully take a leaf from New Scientist about how to moderate. (For example, the NS has no time or patience for those persistent conspiracy theorists who clutter up its comments, and a good thing too.)I received an electronic galley proof of the Telegraph/Eon opening gambit, scheduled for
I'm not giving any secrets away by telling you that the central message of the exercise is that energy companies like E.on are confronted with what they call a "trilemma". (A parallel campaign on YouTube whence came my graphics is already underway with that questionable t-word neologism in prominence). The trilemma refers to so-called "conflicting" demands that future energy supplies be: 1. Green (low-carbon) 2. Affordable and 3. Reliable.
Well, one could be pedantic and say there's nothing conflicting there - they are merely three desirable criteria that need to be met simultaneously. Otherwise our lives would be burdened with trilemma, like where to live (it's got to be a nice area, affordable, handy for public transport).
Rather than nitpick, this blogger decided to focus on renewable energy, and how it might be made more reliable, given fluctuations in wind speed affecting turbine output etc. Clue: Dinorwig.
I'll let the Telegraph publish first, then wait a day or two.
Watch this space!
* Cruel Clever Cat by Geoffrey Taylor:
Sally, having swallowed cheese,
Directs down holes the scented breeze,
Enticing thus with baited breath
Nice mice to an untimely death.
Directs down holes the scented breeze,
Enticing thus with baited breath
Nice mice to an untimely death.
Additional reading E.on powers down to cut carbon and costs
Interesting to see the spotlight being put on our PCs - and rightly so, when you consider the heat that comes - not just from the laptop itself, but the charger too.
Energy-saving tip: when using one's laptop, switch off a light or other appliance that would normally be on, if it's superfluous to requirements when you are online.
Update: Thur 5th November: Here's a C&P of my first post on the E.on site. Having been up for a few days now, it would not seem a terrible breach of protocol to add it here for archival purposes:
Who can doubt that the future lies with renewable energy, and that we Brits are blessed with the stuff – existing or yet-to-be-realized.
First, there are those wind turbines – not the stuff of Wordsworthian rapture I grant you - but they are increasingly being sited offshore.
And there’s wave power – which is a kind of secondhand solar power, recalling that weather and wind are due to unequal heating of the Earth’s surface.
And there’s even the dear old man in the moon, not wishing to be outshone by his flashy big brother, who contributes the prospect of tidal power. Just wait until we have a hydroelectric barrage across the Severn Estuary, supplying maybe as much as 10 per cent (?) of our power supply. (Yes, there are downsides, needless to say, to any big scheme, in terms of amenity, effect on wildlife, capital cost, the carbon-footprint of setting up etc. But let’s stick with the broad brush today.)
The problem with most of the renewable schemes is that the end-product – electricity, that energy-carrier par excellence – is generated at scattered locations across the country, supply may be intermittent, or supply may not match demand around the clock or calendar.
Is there a solution? Yes, there probably is, though it’s not always a panacea. One is talking about big money upfront, and, more to the point, big commitment.
But unless or until fusion power becomes a reality – which may take decades, centuries even – then there is no Plan B, assuming one is not a unbudgeable climate change denialist who thinks the world's scientists in their droves have abandoned all reason in condemning those fossil fuels.
So what is the solution? Simply go to the wiki page on Pumped Hydroelectric Storage, and it’s all there.
Britain already has 4 PHS stations – two in Scotland, two in Wales, and now needs a lot more in different shapes and sizes.
The principle is simple. One has two bodies of water – a lower and upper level. When there’s a surplus of electrical power, say from wind farms during the night, water is pumped from the lower to the upper level. When there’s extra demand, and the conventional stations are struggling to cope, water runs back through turbines, generating electricity.
It’s the closest one can get to “storing” electricity as the potential energy of a head of water. What’s more, the efficiency is surprisingly high – 80 per cent or more they claim in a well-designed system.
Do read the article, to see the new and imaginative ways of developing the principle. The Japanese have used the sea on Okinawa as one of the two levels, the other being a reservoir at the top of the headland.
The Danes have a plan that does not even need two levels – the water is simply pumped into a giant bladder which gradually plumps up, creating its own head. Sand is laid on top to get extra oomph.
My favourite is the salt-mine idea. We have lots of worked-out salt-mines in Cheshire and elsewhere. You pump water down into the old-workings, and site your upper reservoir on the surface. Yes, the water becomes brine, so all the equipment has to be corrosion-resistant. But there’s an upside too: once the water becomes saturated brine, it’s 20 per cent heavier than pure water, so becomes a more efficient energy-transfer medium.
What is it they say – where there’s a will, there’s a way!
Labels:
AGW,
baited breath,
E.on,
eon,
renewable energy,
talking energy,
telegraph,
trilemma
Tuesday, October 20, 2009
Can entropy decrease in a Big Crunch - without defying the Second Law of Thermodynamics?
The Universe, we're told, is expanding, and has been from the beginning of time - reckoned to be some 13.6 billion years ago. Extrapolate back, and the Universe must have started as something incredibly small, hot and dense - a singularity. Something caused that singularity to explode, in a Big Bang. So far, I'm telling you nothing you have not already heard or read many times.
Will the Universe go on expanding for ever? If you believe in Dark Matter and Dark Energy, then the answer is probably yes. But so far, neither of those hypothetical entities has yet been detected.
So there's another scenario that cannot be dismissed - that expansion will slow, and the Universe will cease expanding and then start collapsing back on itself, ending in a Big Crunch.
Some, myself included, are attracted to this idea, especially as it makes possible the idea of a new Big Bang, indeed, a never-ending series of Bangs and Crunches.
But some objections, or at any rate difficulties, have been raised with the idea of a Big Crunch. One of them is to do with entropy (eg link to Yahoo forum) , and the Second Law of Thermodynamics, which is the one I intend to discuss briefly here today and tomorrow.
The essential idea conveyed by the Second Law is that while energy is never created or destroyed, energy is gradually dispersed, becoming less and less useful. Engines use concentrated energy - fuel - to operate. The end product is waste heat - too dispersed for it to be recaptured and re-used. Indeed, the very act of trying to do that would be self-defeating, incurring a greater energy cost than that recouped. Entropy - the spontaneous tendency for systems to become chaotic, more dispersed, has been successfully analysed statistically in terms of order/disorder, more specifically to do with numbers of possible arrangements. The example I used to give students was this. Imagine you have a neat and tidy bedroom, and there's a strong gust of wind through an open window. Papers get scattered, things fall off shelves etc. Suppose one started with a disordered bedroom, and there was a gust of wind. You would be very surprised if you ended with a tidy bedroom. The probability of a chance event - in this case the wind - producing disorder is hugely greater than that of producing order. Why? Because there are relatively few ordered arrangements compared to the number of disordered ones.
What's all this got to do with cosmology you may ask? Well, we see entropy increase around us on a daily basis - eg salt dissolves in water. The ordered structure of a crystal is replaced with the chaos of dispersed ions in solution. If entropy is steadily increasing, in accordance with the Second Law, then the entropy of the initial singularity must presumably have been minimal, possibly zero - a maximally-ordered system it would seem.
There's a problem, then, with the idea of contraction back to a singularity - the Big Crunch. Why ? Because if the end result is the same singularity, then entropy would decrease steadily during the contraction. But that would be contrary to the Second Law, would it not? Other objections have been raised. If we lived in a contracting universe, salt would presumably still dissolve in water, so we would still be seeing the Second Law in action.
Some have tried to get round the conundrum by introducing the variable of time. It then gets very counter-intuitive, especially the concept of negative time, even history running in reverse! Let's not go there.
I believe there is a way of reconciling the concept of a Big Crunch with entropy and the Second Law.
I shall be posting it here tomorrow!
Wed 21 Oct: Well, tomorrow has arrived, so here's the rest of the story.
It's all to do with the size of the Universe, and its fitness or otherwise to act as an entropy-increasing heat sink. While the Universe is expanding, there is abundant space in which heat can dissipate, or other forms of disorder can occur - eg dilution of gaseous end products etc. In the initial stages of contraction, things would continue much the same while there are still light-years between galaxies, or light-minutes between planets and their nearest neighbours, or even light-seconds between a planet and its moon with intervening space.
But imagine the process of contraction occurring continuously. There will finally come a time when one's perception of nature will change. Galaxies will collide for a start, but let's focus on events at a more local level. Previously there was almost limitless space for heat to dissipate. That will no longer be the case - for two reasons. First there is less space for any new heat to dissipate. Secondly, and more importantly, all the previous heat dissipated into the Universe - which is still out there- will become progressively concentrated. (Reminder; it's not just the contents of space that disappear into a black hole vortex - but the fabric of space-time itself- represented by the mesh in the graphic).
Temperatures in deep space, presently a few degree above absolute zero, will start to increase. The so-called microwave background radiation, a left-over from the Big Bang - will gradually shift and start to shorten in wavelength - first to normal radio frequencies, and then into the infra-red region. That's when things start to get interesting. Engines will no longer run so efficiently, because as background temperatures rise, they will find it progressively harder to dissipate exhaust heat.
Let's now look at the salt/water system. Yes, salt will continue to dissolve in water, suggesting that all is well - that the Second Law is still operating. But as background temperatures increase, the water gets hotter, and if there were still observers around, a point would be reached when the water was no longer liquid at normal temperatures and pressures. In other words, salt could not dissolve in water - if there were no liquid water still in existence!
So there would in fact be a gradual violation of the Second Law as we know it, were the Universe to implode towards a Big Crunch, due to increasing difficulty in dissipating waste heat against a background of rising temperature. In the final stages, the temperatures would become so great that no heat could be dissipated at all. In that situation, one has returned to a state of minimum entropy, but hugely elevated temperatures.
Had a classical thermodynamicist such as Carnot (of eponymous cycle fame) been born into a contracting Universe he would have enunciated the Second Law of Thermodynamics differently, methinks. Quite how it would have been worded I would not care to speculate, except to say it would need to have been heavily qualified re differences between open and closed systems. Could a contracting Universe even be described as "open". Only when the system under study was small, with a sizeable temperature difference between it and everything else "out there"?
What then? See my earlier ideas in the margin (scroll down) which have now been appeared in the MSM - so far with no serious objections being raised. I do not believe that the Big Crunch continues indefinitely. There comes a point when, through frictional forces, the plasma reaches the maximum possible temperature - when its constituent particles (strings?) then moving/vibrating so rapidly that they reach the speed of light, and then transform into massless photons. When that occurs, the system ceases to be a superblack hole, and spectacularly flies apart, creating a new Big Bang...
Update 16th Dec 2014 (5 years later!)
It's temperature that is the key to the conundrum, and the kind of world it creates for those seeking evidence of order/disorder.
In our relatively low temperature world (relying on radiated heat from a single sun that is 92 million miles away) we see lots of evidence of order, notably as the presence of substances as liquids and solids, when at higher temperatures (say in the laboratory) they becomes gases, and at thousands or millions of degrees would be in the plasma state.
But the latter are the temperatures that attain when a Universe contracts down to a black hole, and then singularity, hypothetically or otherwise. So it's useless to go looking for the kind of ordered, low entropy signatures that we are accustomed to. We have to ask ourselves what the signatures are when temperatures are hugely elevated, such that subatomic particles are travelling at speeds close to those of light, and colliding with each other. Those collisions break down the order of associations, but progressively a simplified plasma emerges in which there are the ultimate particles only, whatever they happen to be, all crushed together. The original translational energy across sizeable distances now becomes progressively constrained to vibrations about fixed positions (as in a classical earthly solid), obviously with enormous oscillation frequencies. Thus a kind of high-temperature/highly ordered/low entropy state does (paradoxically perhaps) become finally achievable, but through initial fragmentation, rather than clumping association. In other words, there's more than one route to a low entropy state, depending on temperature.
Will the Universe go on expanding for ever? If you believe in Dark Matter and Dark Energy, then the answer is probably yes. But so far, neither of those hypothetical entities has yet been detected.
So there's another scenario that cannot be dismissed - that expansion will slow, and the Universe will cease expanding and then start collapsing back on itself, ending in a Big Crunch.
Some, myself included, are attracted to this idea, especially as it makes possible the idea of a new Big Bang, indeed, a never-ending series of Bangs and Crunches.
But some objections, or at any rate difficulties, have been raised with the idea of a Big Crunch. One of them is to do with entropy (eg link to Yahoo forum) , and the Second Law of Thermodynamics, which is the one I intend to discuss briefly here today and tomorrow.
The essential idea conveyed by the Second Law is that while energy is never created or destroyed, energy is gradually dispersed, becoming less and less useful. Engines use concentrated energy - fuel - to operate. The end product is waste heat - too dispersed for it to be recaptured and re-used. Indeed, the very act of trying to do that would be self-defeating, incurring a greater energy cost than that recouped. Entropy - the spontaneous tendency for systems to become chaotic, more dispersed, has been successfully analysed statistically in terms of order/disorder, more specifically to do with numbers of possible arrangements. The example I used to give students was this. Imagine you have a neat and tidy bedroom, and there's a strong gust of wind through an open window. Papers get scattered, things fall off shelves etc. Suppose one started with a disordered bedroom, and there was a gust of wind. You would be very surprised if you ended with a tidy bedroom. The probability of a chance event - in this case the wind - producing disorder is hugely greater than that of producing order. Why? Because there are relatively few ordered arrangements compared to the number of disordered ones.
What's all this got to do with cosmology you may ask? Well, we see entropy increase around us on a daily basis - eg salt dissolves in water. The ordered structure of a crystal is replaced with the chaos of dispersed ions in solution. If entropy is steadily increasing, in accordance with the Second Law, then the entropy of the initial singularity must presumably have been minimal, possibly zero - a maximally-ordered system it would seem.
There's a problem, then, with the idea of contraction back to a singularity - the Big Crunch. Why ? Because if the end result is the same singularity, then entropy would decrease steadily during the contraction. But that would be contrary to the Second Law, would it not? Other objections have been raised. If we lived in a contracting universe, salt would presumably still dissolve in water, so we would still be seeing the Second Law in action.
Some have tried to get round the conundrum by introducing the variable of time. It then gets very counter-intuitive, especially the concept of negative time, even history running in reverse! Let's not go there.
I believe there is a way of reconciling the concept of a Big Crunch with entropy and the Second Law.
I shall be posting it here tomorrow!
Wed 21 Oct: Well, tomorrow has arrived, so here's the rest of the story.
It's all to do with the size of the Universe, and its fitness or otherwise to act as an entropy-increasing heat sink. While the Universe is expanding, there is abundant space in which heat can dissipate, or other forms of disorder can occur - eg dilution of gaseous end products etc. In the initial stages of contraction, things would continue much the same while there are still light-years between galaxies, or light-minutes between planets and their nearest neighbours, or even light-seconds between a planet and its moon with intervening space.
But imagine the process of contraction occurring continuously. There will finally come a time when one's perception of nature will change. Galaxies will collide for a start, but let's focus on events at a more local level. Previously there was almost limitless space for heat to dissipate. That will no longer be the case - for two reasons. First there is less space for any new heat to dissipate. Secondly, and more importantly, all the previous heat dissipated into the Universe - which is still out there- will become progressively concentrated. (Reminder; it's not just the contents of space that disappear into a black hole vortex - but the fabric of space-time itself- represented by the mesh in the graphic).
Temperatures in deep space, presently a few degree above absolute zero, will start to increase. The so-called microwave background radiation, a left-over from the Big Bang - will gradually shift and start to shorten in wavelength - first to normal radio frequencies, and then into the infra-red region. That's when things start to get interesting. Engines will no longer run so efficiently, because as background temperatures rise, they will find it progressively harder to dissipate exhaust heat.
Let's now look at the salt/water system. Yes, salt will continue to dissolve in water, suggesting that all is well - that the Second Law is still operating. But as background temperatures increase, the water gets hotter, and if there were still observers around, a point would be reached when the water was no longer liquid at normal temperatures and pressures. In other words, salt could not dissolve in water - if there were no liquid water still in existence!
So there would in fact be a gradual violation of the Second Law as we know it, were the Universe to implode towards a Big Crunch, due to increasing difficulty in dissipating waste heat against a background of rising temperature. In the final stages, the temperatures would become so great that no heat could be dissipated at all. In that situation, one has returned to a state of minimum entropy, but hugely elevated temperatures.
Had a classical thermodynamicist such as Carnot (of eponymous cycle fame) been born into a contracting Universe he would have enunciated the Second Law of Thermodynamics differently, methinks. Quite how it would have been worded I would not care to speculate, except to say it would need to have been heavily qualified re differences between open and closed systems. Could a contracting Universe even be described as "open". Only when the system under study was small, with a sizeable temperature difference between it and everything else "out there"?
What then? See my earlier ideas in the margin (scroll down) which have now been appeared in the MSM - so far with no serious objections being raised. I do not believe that the Big Crunch continues indefinitely. There comes a point when, through frictional forces, the plasma reaches the maximum possible temperature - when its constituent particles (strings?) then moving/vibrating so rapidly that they reach the speed of light, and then transform into massless photons. When that occurs, the system ceases to be a superblack hole, and spectacularly flies apart, creating a new Big Bang...
Update 16th Dec 2014 (5 years later!)
It's temperature that is the key to the conundrum, and the kind of world it creates for those seeking evidence of order/disorder.
In our relatively low temperature world (relying on radiated heat from a single sun that is 92 million miles away) we see lots of evidence of order, notably as the presence of substances as liquids and solids, when at higher temperatures (say in the laboratory) they becomes gases, and at thousands or millions of degrees would be in the plasma state.
But the latter are the temperatures that attain when a Universe contracts down to a black hole, and then singularity, hypothetically or otherwise. So it's useless to go looking for the kind of ordered, low entropy signatures that we are accustomed to. We have to ask ourselves what the signatures are when temperatures are hugely elevated, such that subatomic particles are travelling at speeds close to those of light, and colliding with each other. Those collisions break down the order of associations, but progressively a simplified plasma emerges in which there are the ultimate particles only, whatever they happen to be, all crushed together. The original translational energy across sizeable distances now becomes progressively constrained to vibrations about fixed positions (as in a classical earthly solid), obviously with enormous oscillation frequencies. Thus a kind of high-temperature/highly ordered/low entropy state does (paradoxically perhaps) become finally achievable, but through initial fragmentation, rather than clumping association. In other words, there's more than one route to a low entropy state, depending on temperature.
Saturday, October 10, 2009
What went wrong with NASA's moon crash?
The idea was to crash a spacecraft into the Moon at a chosen spot where ice might be lurking. Hitting the Moon at twice the speed of a bullet would send a plume of debris miles high that could then be analysed spectroscopically for water.
But there appears to have been no plume - only a brief white flash. Why not? Here's this blogger's explanation for what it's worth, published first on New Scientist and now the Times.
From the latter:
"Maybe a plume was formed but settled too quickly to be seen by the following probe, 4 minutes behind. Don't forget that although gravity is lower on the Moon, making things slower to fall, there's no air to slow the descent - even of dust particles or those hoped-for ice crystals.
There's a classic lab experiment in which a stout glass tube has all the air pumped out. A feather inside then falls as quickly as a ball-bearing.
Let's hope the NASA scientists did not forget their school physics."
Update: Sunday 18 October Well, halleluja, a plume was captured on camera after all, although the photograph in New Scientist ("Elusive lunar plume caught on camera after all") was somewhat disappointing. I mean to say - given it was said to be 6-8 kilometres wide, why show us a mere smidgeon of white on a so-called "zoomed image"? Small wonder the conspiracy theorists began falling out of the woodwork!
The fact that a plume was briefly formed lends support to the hypothesis I've ventured above. The plume didn't last long, because the particles fell back faster than many might suppose (given that the Moon's lower gravity seems to dominate most thinking, with the absence of an atmosphere frequently overlooked).
The absence of an atmosphere alters the dynamics of an impact and its aftermath considerably. Dust and other particles (ice?) may well be ejected much higher and further than on Earth, due to absence of a cushioning atmosphere - no air molecules to be pushed aside- but their return to "earth" would look entirely different to an observer or camera. Initially the descent would seem gentle - due to 1/6th Earth's gravity- but acceleration would be continuous, without anything comparable to "terminal velocity" (approx 120 mph on Earth in "old money"). A quick back- of- envelope calculation using school physics says that while it would take some 30 seconds for dust or ice particles to reach 120mph (which is our earthly terminal velocity), they would go on accelerating indefinitely until they finally hit the surface. There's another factor to consider - which another scribe on NS comments has pointed out : much of the ice -if present- would be vaporised by the kinetic energy (heat) of impact. The molecules would be unlikely to re-coalesce in the Moon's near-vacuum, and simply disappear from view - except perhaps to a highly sensitive spectrometer that was set up specifically to look for them - and certainly fail to drop back. Molecules generally don't "drop back" in a vacuum, especially in a weak gravitational field. They would simply spread out, ie diffuse rapidly.
But there appears to have been no plume - only a brief white flash. Why not? Here's this blogger's explanation for what it's worth, published first on New Scientist and now the Times.
From the latter:
"Maybe a plume was formed but settled too quickly to be seen by the following probe, 4 minutes behind. Don't forget that although gravity is lower on the Moon, making things slower to fall, there's no air to slow the descent - even of dust particles or those hoped-for ice crystals.
There's a classic lab experiment in which a stout glass tube has all the air pumped out. A feather inside then falls as quickly as a ball-bearing.
Let's hope the NASA scientists did not forget their school physics."
Update: Sunday 18 October Well, halleluja, a plume was captured on camera after all, although the photograph in New Scientist ("Elusive lunar plume caught on camera after all") was somewhat disappointing. I mean to say - given it was said to be 6-8 kilometres wide, why show us a mere smidgeon of white on a so-called "zoomed image"? Small wonder the conspiracy theorists began falling out of the woodwork!
The fact that a plume was briefly formed lends support to the hypothesis I've ventured above. The plume didn't last long, because the particles fell back faster than many might suppose (given that the Moon's lower gravity seems to dominate most thinking, with the absence of an atmosphere frequently overlooked).
The absence of an atmosphere alters the dynamics of an impact and its aftermath considerably. Dust and other particles (ice?) may well be ejected much higher and further than on Earth, due to absence of a cushioning atmosphere - no air molecules to be pushed aside- but their return to "earth" would look entirely different to an observer or camera. Initially the descent would seem gentle - due to 1/6th Earth's gravity- but acceleration would be continuous, without anything comparable to "terminal velocity" (approx 120 mph on Earth in "old money"). A quick back- of- envelope calculation using school physics says that while it would take some 30 seconds for dust or ice particles to reach 120mph (which is our earthly terminal velocity), they would go on accelerating indefinitely until they finally hit the surface. There's another factor to consider - which another scribe on NS comments has pointed out : much of the ice -if present- would be vaporised by the kinetic energy (heat) of impact. The molecules would be unlikely to re-coalesce in the Moon's near-vacuum, and simply disappear from view - except perhaps to a highly sensitive spectrometer that was set up specifically to look for them - and certainly fail to drop back. Molecules generally don't "drop back" in a vacuum, especially in a weak gravitational field. They would simply spread out, ie diffuse rapidly.
Labels:
moon crash,
NASA,
search for lunar water
Thursday, October 8, 2009
The Times "Eureka" magazine - first impressions
I've just spent an hour leafing through the Times's most recent addition to its publishing platform. It's called Eureka, its cover is very green, with an unattractive graphic of a chap who's lost the top half of his skull, with a plethora of sciencey things bursting forth from the exposed remainder.
Personally I find it hard to conceive of an image that is better guaranteed to confirm in the layman's mind that science is a nerdy thing that reveals more than you really want to know - an autistic lack of the light touch, to say nothing of discretion and good taste .
Well, I hadn't intended to start on so negative a note, but it's sitting there next to me in all its disturbing, stomach-churning immediacy, so I want to get that off my chest. First impressions still count, do they not?
Second and subsequent impressions are a lot more favourable. One doesn't envy anyone the task of producing a digest of science for the general reader of a newspaper, not even a serious one like The Times. How does one define the target audience, given that a relatively small proportion will have formal scientific qualifications much beyond GCSE - or O-Level? Then there is the notorious antipathy that exists towards science and scientists in the UK - one that has produced what someone described some years ago as the "ghettoization" of science.
Scientists are partly to blame themselves - let's not go into all that right now. Suffice it to say that the finger hovers over the remote when a science programme appears on TV. Woe betide any presenter who attempts to get too immersed in detail, or who overdoes the gee-whizzery, pie-in-the-sky delivery. The British public is inured to that kind of thing, and is likely to say "Come back in 10 years when you know some more, or have a workable, marketable product."
The criterion for a good thriller is that it is un-putdown-able. So what should it be for a periodical that appears only once a month? Not un-putdownable - that would be asking too much, in view of the subject matter. I suggest it should be put-downable, ie nothing too long and demanding of time, but so pick-uppable again that it escapes an early fate in the nether regions of a bulging paper-rack, or worse still the dustbin. In that sense I believe "Eureka" is over its first hurdle. There's a good mix of light and serious, human and technical, visionary and realistic to make it worth returning to if one only has the time or inclination to dip in now and again.
I'll come back in a day or two with a closer look at some of the features. I do have one or two quite serious gripes with some of the detailed science. In particular the feature "Living in the City" seems to have some unrealistic chemistry and biology. Since when has carbon dioxide reacted with magnesium chloride to give magnesium carbonate? (The reaction proceeds fine in the opposite direction!) But let's not nitpick today. The line-up of writers is impressive - with at least three with solid research experience and qualifications - up to and beyond PhD - and amazingly the Times has secured the services of Martin Rees - President of the Royal Society- whose keynote contribution is worth a read, touching as it does on a host of the issues that confront scientists, and the perception (or misconceptions) re the scientific approach to modern life and the myriad questions it throws up.
Update: Friday 9 October
The individual articles in Eureka are now online, including the one with the questionable chemistry. Have submitted this comment:
"Hmmm. While one welcomes "out-of-the-box" thinking on technology, the fundamental science has to stay firmly within the box.
It would not seem feasible, for example, to use "magnesium chloride" as a trapping agent for CO2. Magnesium hydroxide, certainly, but since that's made from magnesium carbonate, heating in steam to drive off CO2, it's self defeating re carbon footprints.
With magnesium chloride, one is trying to make the following (well known) reaction go in reverse:
magnesium carbonate + hydrochloric acid -> magnesium chloride + water + carbon dioxide
Not only does it prefer to go in the direction shown, but if one did contrive conditions to make it go in reverse one would release hydrochloric acid fumes into the neighbourhood! Hardly environmentally-friendly!
The bacterial illumination look improbable too. Yes, there are strains that react oxygen with luciferin, but the light-show is mediated by the enzyme luciferase, and that catalysis, like the rest of bacterial metabolism, requires an aqueous environment. I doubt whether bacteria would take kindly to being incorporated into coatings if that meant having to exist lichen-like in all weathers. Bacteria - excluding their resistant spores- are a lot more fussy about their environment!"
Personally I find it hard to conceive of an image that is better guaranteed to confirm in the layman's mind that science is a nerdy thing that reveals more than you really want to know - an autistic lack of the light touch, to say nothing of discretion and good taste .
Well, I hadn't intended to start on so negative a note, but it's sitting there next to me in all its disturbing, stomach-churning immediacy, so I want to get that off my chest. First impressions still count, do they not?
Second and subsequent impressions are a lot more favourable. One doesn't envy anyone the task of producing a digest of science for the general reader of a newspaper, not even a serious one like The Times. How does one define the target audience, given that a relatively small proportion will have formal scientific qualifications much beyond GCSE - or O-Level? Then there is the notorious antipathy that exists towards science and scientists in the UK - one that has produced what someone described some years ago as the "ghettoization" of science.
Scientists are partly to blame themselves - let's not go into all that right now. Suffice it to say that the finger hovers over the remote when a science programme appears on TV. Woe betide any presenter who attempts to get too immersed in detail, or who overdoes the gee-whizzery, pie-in-the-sky delivery. The British public is inured to that kind of thing, and is likely to say "Come back in 10 years when you know some more, or have a workable, marketable product."
The criterion for a good thriller is that it is un-putdown-able. So what should it be for a periodical that appears only once a month? Not un-putdownable - that would be asking too much, in view of the subject matter. I suggest it should be put-downable, ie nothing too long and demanding of time, but so pick-uppable again that it escapes an early fate in the nether regions of a bulging paper-rack, or worse still the dustbin. In that sense I believe "Eureka" is over its first hurdle. There's a good mix of light and serious, human and technical, visionary and realistic to make it worth returning to if one only has the time or inclination to dip in now and again.
I'll come back in a day or two with a closer look at some of the features. I do have one or two quite serious gripes with some of the detailed science. In particular the feature "Living in the City" seems to have some unrealistic chemistry and biology. Since when has carbon dioxide reacted with magnesium chloride to give magnesium carbonate? (The reaction proceeds fine in the opposite direction!) But let's not nitpick today. The line-up of writers is impressive - with at least three with solid research experience and qualifications - up to and beyond PhD - and amazingly the Times has secured the services of Martin Rees - President of the Royal Society- whose keynote contribution is worth a read, touching as it does on a host of the issues that confront scientists, and the perception (or misconceptions) re the scientific approach to modern life and the myriad questions it throws up.
Update: Friday 9 October
The individual articles in Eureka are now online, including the one with the questionable chemistry. Have submitted this comment:
"Hmmm. While one welcomes "out-of-the-box" thinking on technology, the fundamental science has to stay firmly within the box.
It would not seem feasible, for example, to use "magnesium chloride" as a trapping agent for CO2. Magnesium hydroxide, certainly, but since that's made from magnesium carbonate, heating in steam to drive off CO2, it's self defeating re carbon footprints.
With magnesium chloride, one is trying to make the following (well known) reaction go in reverse:
magnesium carbonate + hydrochloric acid -> magnesium chloride + water + carbon dioxide
Not only does it prefer to go in the direction shown, but if one did contrive conditions to make it go in reverse one would release hydrochloric acid fumes into the neighbourhood! Hardly environmentally-friendly!
The bacterial illumination look improbable too. Yes, there are strains that react oxygen with luciferin, but the light-show is mediated by the enzyme luciferase, and that catalysis, like the rest of bacterial metabolism, requires an aqueous environment. I doubt whether bacteria would take kindly to being incorporated into coatings if that meant having to exist lichen-like in all weathers. Bacteria - excluding their resistant spores- are a lot more fussy about their environment!"
Labels:
Martin Rees,
Times Eureka
Friday, September 25, 2009
That so-called "Anglo-Saxon" treasure could have been of genuine British-manufacture
I'm going to stick my neck out here. Who's to say that the fabulous hoard of gold and silver craftwork, unearthed by that bloke with the metal detector in Staffordshire, was produced by Johnny-Come-Lately Anglo-Saxon settlers?
OK, so we are supposed to be proud of our admixture of Anglo-Saxon and other genes. We are supposed to take pride in being an allegedly mongrel race (hybrid vigour an' all).
But have your read your Stephen Oppenheimer? He's the Oxford scholar who analyses modern DNA to trace our genetic roots. I blogged about his claims some 3 years ago. Oppenheimer rejects the idea that Anglo-Saxons supplanted the native Brits, sending us scurrying to the Celtic fringes. Oppenheimer reckons that the native Brits pre-dated the Celts, and indeed resisted numerous waves of invasion - the Celts, the Romans, the Anglo-Saxons, the Normans, stubbornly staying put, and surviving - either by putting up resistance, or by assimilating the invaders.
Oppenheimer reckons that native Brits are derived from Basque migrants who recolonised Britain some 15,000 years ago after the last Ice Age, crossing the then still existing land bridge between the Continent and England.
So why is the treasure being described as Anglo-Saxon? Who's to say it is not native British - or, less probably, Celt?
OK, so it was discovered in a Staffordshire field, in central England, and is reckoned to be 7th century, when that part of England was in the Anglo-Saxon kingdom of Mercia. But that does not mean that it was Anglo-Saxon treasure. It may have been of native British manufacture, possibly from the era (mythical or otherwise) of King Arthur, Camelot, and the Knights of the Round Table. It may have been taken from our dead warriors on the battlefield as war booty - or maybe our knights buried their finery (purely for show!) before facing the invader in battle wearing the equivalent of combat fatigues.
What's the evidence that Anglo-Saxons ever produced such exquisite artwork? I thought they were practical types, more concerned with clearing forest, ploughing, agriculture and animal husbandry? Their efforts went into producing axes and ploughs - not fine gold filigree ornamentation.
Yep, I'll put my head on the block. The "experts" have got it entirely wrong. It's not Anglo-Saxon treasure. It's British treasure. And no, I don't mean Celtic treasure - although that gifted if mercurial folk did produce fine arts and crafts - and are arguably more highly regarded in that respect than the Anglo-Saxons.
Nope, I reckon the treasure was produced by native "aboriginal" Brits, of Basque-derived stock according to Professor Oppenheimer - the true Brits - the ones who re-settled the British Isles, and who have managed to survive and prosper despite waves of immigrants seeking a better life. Britain's geography permitted co-existence within probably quite confined geographical areas - a few hundred square miles for example - which could be a little as 20m x 20m- thanks to our varied topography, the (then) more abundant forest cover, and, dare I say it, mutual tolerance and/or respect between native and newcomer. There was room for everyone who was prepared to work, and to "live and let live" - the British way. Britain was, if you like, the "New World" of the first millennium, once the Romans had left.
I repeat: the experts may have got it wrong. One cannot assume it is Anglo-Saxon treasure. It could well have been the exquisite handiwork of native Brits who succeeded in maintaining their genetic, ethnic and cultural identity over thousands of years - standing firm and finally expelling - or assimilating- the Celts, Romans, Anglo-Saxons, Danes, Vikings and Normans.
Update Sat 26 14:31 : See the Telegraph article with its 23 comments - at the time of writing.
The "Staffordshire Hoard" now has a wikipedia entry
Just 9 of some 1500 so-called "Anglo-Saxon" artefacts - a stupendous find, regardless of provenance
OK, so we are supposed to be proud of our admixture of Anglo-Saxon and other genes. We are supposed to take pride in being an allegedly mongrel race (hybrid vigour an' all).
But have your read your Stephen Oppenheimer? He's the Oxford scholar who analyses modern DNA to trace our genetic roots. I blogged about his claims some 3 years ago. Oppenheimer rejects the idea that Anglo-Saxons supplanted the native Brits, sending us scurrying to the Celtic fringes. Oppenheimer reckons that the native Brits pre-dated the Celts, and indeed resisted numerous waves of invasion - the Celts, the Romans, the Anglo-Saxons, the Normans, stubbornly staying put, and surviving - either by putting up resistance, or by assimilating the invaders.
Oppenheimer reckons that native Brits are derived from Basque migrants who recolonised Britain some 15,000 years ago after the last Ice Age, crossing the then still existing land bridge between the Continent and England.
So why is the treasure being described as Anglo-Saxon? Who's to say it is not native British - or, less probably, Celt?
OK, so it was discovered in a Staffordshire field, in central England, and is reckoned to be 7th century, when that part of England was in the Anglo-Saxon kingdom of Mercia. But that does not mean that it was Anglo-Saxon treasure. It may have been of native British manufacture, possibly from the era (mythical or otherwise) of King Arthur, Camelot, and the Knights of the Round Table. It may have been taken from our dead warriors on the battlefield as war booty - or maybe our knights buried their finery (purely for show!) before facing the invader in battle wearing the equivalent of combat fatigues.
What's the evidence that Anglo-Saxons ever produced such exquisite artwork? I thought they were practical types, more concerned with clearing forest, ploughing, agriculture and animal husbandry? Their efforts went into producing axes and ploughs - not fine gold filigree ornamentation.
Yep, I'll put my head on the block. The "experts" have got it entirely wrong. It's not Anglo-Saxon treasure. It's British treasure. And no, I don't mean Celtic treasure - although that gifted if mercurial folk did produce fine arts and crafts - and are arguably more highly regarded in that respect than the Anglo-Saxons.
Nope, I reckon the treasure was produced by native "aboriginal" Brits, of Basque-derived stock according to Professor Oppenheimer - the true Brits - the ones who re-settled the British Isles, and who have managed to survive and prosper despite waves of immigrants seeking a better life. Britain's geography permitted co-existence within probably quite confined geographical areas - a few hundred square miles for example - which could be a little as 20m x 20m- thanks to our varied topography, the (then) more abundant forest cover, and, dare I say it, mutual tolerance and/or respect between native and newcomer. There was room for everyone who was prepared to work, and to "live and let live" - the British way. Britain was, if you like, the "New World" of the first millennium, once the Romans had left.
I repeat: the experts may have got it wrong. One cannot assume it is Anglo-Saxon treasure. It could well have been the exquisite handiwork of native Brits who succeeded in maintaining their genetic, ethnic and cultural identity over thousands of years - standing firm and finally expelling - or assimilating- the Celts, Romans, Anglo-Saxons, Danes, Vikings and Normans.
Update Sat 26 14:31 : See the Telegraph article with its 23 comments - at the time of writing.
The "Staffordshire Hoard" now has a wikipedia entry
Thursday, September 24, 2009
No genuinely free water on the Moon, it would seem...
The ambiguity of "free" in my title is deliberate. The traces of water detected by that inspired Indian lunar probe are not of course free water, in a chemical sense, but chemically-bound, non-wettening "water".
Even my non-scientific wife was quick to realize that, watching last night's news, even if the media reports elsewhere conjure up visions of future moon colonists tapping into an abundant supply. Moonshine!
Being chemically-bound, whether weakly or strongly, it's hardly free for the taking either. The expression "getting blood out of a stone" springs to mind.
Here's what I sent last night to the Times in response to that trumpeting headline re there being a litre of water per tonne of lunar soil. (One feels that "water" should have been enclosed in quotation marks in the Times's article).
Is that a water-diviner in his hand? If so, then happy-hunting..
Being chemically-bound, whether weakly or strongly, it's hardly free for the taking either. The expression "getting blood out of a stone" springs to mind.
Here's what I sent last night to the Times in response to that trumpeting headline re there being a litre of water per tonne of lunar soil. (One feels that "water" should have been enclosed in quotation marks in the Times's article).
September 24, 2009 11:10 PM BST on community.timesonline.co.uk
"Hydroxyl", note, is not a molecule, as the report would have one believe. Being simply OH, with an unpaired electron, it would be a free radical, with no independent existence, and a lifetime of seconds at most if generated in the atmosphere by, say, cosmic ray bombardment. Most "OH" in rocks is, of course, present as negatively-charged hydroxyl ions, accompanying positively-charged metal ions, eg aluminium.
Aside: well done, btw, Times for finally adding a time and date stamp to readers' comments, and for a much-speedier moderation than before. The BBC-style facility for expressing approval is also cute. It even survives cut-and-pasting here, apparently as a live-link - see the blue font above! Shame though that one's comments are not accessible to search engines. The world wide web depends on linking, you know, if only for information-retrieval. Vanity has nowt to do with it, of course...
Update: Friday 15:15 pm. Had some further thoughts on the inconclusive nature of the chemistry after reading the New Scientist's feature: Have just sent the following:
"Hmmm. So the signal could have come from water or from the "OH molecule". Leaving aside the faulty nomenclature - OH is not a molecule, but is either a free radical if electrically neutral, or a negatively- charged ion - it seems a bit of a liberty to lump together two entirely different chemical species in this manner. The discovery of H2O would indeed be exciting, even if strongly adsorbed to minerals. But "hydroxyl", presumably as mineral hydroxides, would be an entirely different matter, requiring somewhat high temperatures in most cases if desiring to drive off molecular water, which would then have to be cooled and condensed. Most of the hydroxides of predominant minerals in the Earth's crust - magnesium, calcium, aluminium etc- hang onto their oxygen and hydrogen quite firmly, needing red heat or higher to dissociate into oxides and steam.
So it's somewhat premature surely to report that "water" has been discovered. On the basis of available evidence, none of which can be described as "hard", what's been discovered are oxygen atoms that are bonded to one or possibly two hydrogen atoms with a strong attachment to a mineral matrix given they are able to survive solar heating in a vacuum. Alternatively, and less usefully from the point of view of harvesting lunar water, the signal is picking up a temporary association, ie the turnover model, which would explain why the discovery did not come earlier from study of Apollo-mission rocks"
Update Sep 25 21:17 : See also "How could astronauts harvest water on the Moon" - latest article in New Scientist.
Update: Friday 15:15 pm. Had some further thoughts on the inconclusive nature of the chemistry after reading the New Scientist's feature: Have just sent the following:
"Hmmm. So the signal could have come from water or from the "OH molecule". Leaving aside the faulty nomenclature - OH is not a molecule, but is either a free radical if electrically neutral, or a negatively- charged ion - it seems a bit of a liberty to lump together two entirely different chemical species in this manner. The discovery of H2O would indeed be exciting, even if strongly adsorbed to minerals. But "hydroxyl", presumably as mineral hydroxides, would be an entirely different matter, requiring somewhat high temperatures in most cases if desiring to drive off molecular water, which would then have to be cooled and condensed. Most of the hydroxides of predominant minerals in the Earth's crust - magnesium, calcium, aluminium etc- hang onto their oxygen and hydrogen quite firmly, needing red heat or higher to dissociate into oxides and steam.
So it's somewhat premature surely to report that "water" has been discovered. On the basis of available evidence, none of which can be described as "hard", what's been discovered are oxygen atoms that are bonded to one or possibly two hydrogen atoms with a strong attachment to a mineral matrix given they are able to survive solar heating in a vacuum. Alternatively, and less usefully from the point of view of harvesting lunar water, the signal is picking up a temporary association, ie the turnover model, which would explain why the discovery did not come earlier from study of Apollo-mission rocks"
Update Sep 25 21:17 : See also "How could astronauts harvest water on the Moon" - latest article in New Scientist.
Subscribe to:
Comments (Atom)


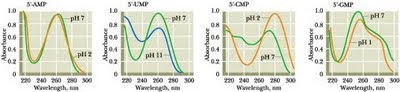


























Why not get the Sahara green first - to create biomass and reduce CO2? Huge problems to overcome, yes, but if we can't lick that problem, what hope is there of colonising the Moon or Mars on a realistic timescale?