Newsflash: Monday 10th October, 2016
Have just come across this item in the Mail.
http://www.dailymail.co.uk/sciencetech/article-3830773/The-secret-ingredient-life-Earth-THICKENER-kick-started-evolution-living-creatures.html
I strongly recommend it, especially as there are faint echoes of the thinking in this posting of mine from way back in 2009.
Start of original posting:
An opportunity has just presented itself to share with my fellow earthlings an idea that's been fermenting in this senescent brain for some time. It concerns the building blocks of DNA and RNA - the purine and pyrimidine bases. These are the flat stackeable molecules that make up the base pairs in double-stranded DNA - Watson and Crick's double helix- and thence the triplet genetic code.
Left: pyrimidines and purines. Right: a pyrimidine nucleotide showing the intermediate ribose sugar between a pyrimidine base and phosphate
Why were these particular molecules selected from the primeval soup? Could other molecules have served in their place? Did adenine and guanine (the purines) and cytosine, thymine and uracil (the pyrimidines) just happen to be "lying around", so to speak, in some primeval rock pool, or forming in the vicinity of a hydrothermal vent?
I tried tackling it first from a physicochemical standpoint. What conditions would be needed for any kind of spontaneous chemistry to occur in a puddle say on Earth? The classic Miller-Urey experiments you may recall subjected the assumed constituents of Earth's proto-atmosphere to electric discharges, in an attempt to generate the precursors of proteins and nucleic acids (amino acids, purines and pyrimidines). Glycine - the simplest of the amino acids- was formed, but that was hardly a chemical cornucopia.
The Miller-Urey apparatus. The test mixture of water, methane, ammonia, hydrogen and carbon monoxide was intended to replicate the then-imagined reducing atmosphere. Current thinking is that the main carbon component was CO2 rather than methane CH4. Interestingly, substituting CO2 for methane gives the same range of amino acid and other products, provided that oxygen is excluded.
See wiki for more details
See wiki for more details
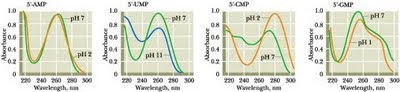
One of the chief problems would have been intense uv radiation from space - without oxygen, there would have been no ozone "sun-screen". So maybe the first requirement was to generate sun-screen agents in situ. Purines and pyrimidines all absorb strongly in the ultraviolet:
From left: adenine ( as the nucleotide AMP ); uracil (as UMP); cytosine (as CMP); guanine (as GMP)
So if they formed by accident a kind of proto-Darwinian natural selection would operate. Further molecules could then evolve, protected by the sun-screen molecules. What about the sugars - the five carbon ribose and deoxyribose? Could they have developed initially in a permissive role, simply to allow more chemistry to proceed?
If life did evolve on land, say in a rock pool receiving intermittent rain, and exposed to all weathers, then there needed to be a mechanism to protect against freezing or evaporation. Chemistry generally needs to be in solution if one is to synthesise. Well, there's a possible role for sugars. Firstly, they depress freezing point, ie have anti-freeze properties. What's more, a sugar solution tends to go syrupy when water is evaporated. It's often quite difficult to drive off the last of the water. Sugar molecules bristle with hydrogen-bonding OH groups which cling tenaciously to water.
Sun-screen agent, anti-freeze - are these the properties of purines, pyrimidines and sugars that caused them to become the building blocks of life?
What about those five chemical bases? Where did the carbon and nitrogen come from for their cyclic structures?
It was the article in the current New Scientist ("Was life founded on cyanide from space crashes?") that provided another piece of the jigsaw. It's proposed that life on earth was seeded by cyanide , -CN, in asteroids. As written it's a free radical, not a stable molecule. It would have to be HCN, or a metal cyanide, eg KCN. Now if you look at the detailed structure of purines and pyrimidines, you will see that all of them feature an alternation of -C-N- in their rings. There are some -C-C bonds as well, but the cyanide dimer cyanogen N-C-C-N could have provided that.
As soon as I started googling I struck pay dirt, with a 1978 paper describing how solutions of HCN began to form purines and pyrimidines spontaneously in the presence of alkali.
Read the New Scientist article and comments, five at the time of writing, with the suggestion that cyanide is the carbon precursor par excellence, not only for its association with nitrogen, but for being "electron-rich" (my term), given the presence of that triple bond between the two atoms, representing three shared pairs of electrons.
Standard valence formula (lower left); electronic configuration (upper right). Of the six electrons in the triple bond, 4 are available for forming new chemical bonds with additional atoms of carbon, nitrogen, hydrogen etc.
Further reading: for a critique of the Miller-Urey experiment, indeed, the entire idea that life could have evolved spontaneously, see the article by John Peet.
Postscript: it's all been thought of before: at least the cyanide to purines route.
And, to end on a light note:
Postscript: added October 8, 2016:
It's nigh on 7 years since penning this post, which still continues to receive a trickle of visitors, according to my sitemeter. Much water has flowed undr this blogger's bridge these last few years. First there's this retired science bod's explanation for the Turin Shroud - a flour/oil imprint from a live adult male (probably 13th century!) onto linen intitially, then roasted, then washed, to produce the final faint, enigmatic 'negative' image. See my specialist TS site for details.
There's also my theory for Stonehenge, and indeed most standing stones, circles and henges in the UK, namely that they were sites for "sky burial", or as I prefer to call it, AFS (avian-facilitated skeletonization). See the most recent postings on this site for the basis of this interpreation, much resting on the crude and rustic layout of what has been dubbed "Seahenge" uncovered by storms on the Norfolk coast in the late 1990s.























1 comment:
Post a Comment