Introduction and Summary: It was supposed initially to be a pot-boiler of a posting, describing what a strong solution of sulphuric acid (H2SO4) i.e. "battery acid" does to linen when it becomes more concentrated by slow unaided evaporation. I needed to know in order to evaluate a current hypothesis regarding the Shroud of Turin and its 'enigmatic' image.
It then turned into something different - and hopefully more significant - when I found different results with cotton from those with linen, and began doing some reading (including my own half-forgotten postings from as long as two years ago).
I shall stick with the original idea of showing the results of my experiments in a series of photographs - that being the raison d'etre of this site - to allow the reader to see things through the eyes of a retired researcher - albeit one who makes no claims to experimental sophistication, restricted as he is to a kitchen (or in this case garage) laboratory, dependent on internet suppliers for chemicals etc. But here first is a short summary for the benefit of those who simply want a take-away message:
Summary: evaporation of initially approx 35% sulphuric acid, H2SO4, on white linen resulted after 2 days or more in loss of mechanical strength, with only slight darkening of the fabric. A cotton sample similarly treated retained its colour and mechanical strength. The difference may be due to the presence in the central cores of linen fibres (secondary cell wall) of appreciable amounts of hemicelluloses that are more chemically reactive than cellulose and essentially absent from cotton.
These results raise the possibility that the difference between the Turin Shroud's coloured image v non-image fibres are NOT confined to the highly superficial primary cell wall (estimated 200-600nm thickness) and may extend to the central core of the fibres too, resulting in loss of physical and chemical integrity, explaining perhaps the relative mechanical weakness of image fibres reported by STURP's Raymond N.Rogers in his sticky tape sampling. If so, the process that gave rise to image imprinting on the TS may not have been as subtle as previously claimed, making it possible perhaps to model using technology that was available in the 14th century, corresponding to the radiocarbon dating.
Now for the experimental details:
 |
| The label says it's "100% linen". It's about to be cut into strips. |
 |
| Strip cut away, with handy ribs that act as guidelines for what follows |
 |
| The strip has been used to make the chemical formula for sulphuric acid. The cable ties are for dipping in battery acid (see unopened container in background). |
 |
| Here's a close-up. It states the specific gravity. One has to assume it's the standard concentration for battery acid (usually around 35% H2SO4) |
 |
| Here's a long-shot of the experiment set up in the garage. Note the polyethylene box to contain any spills. |
 |
| Ready for dipping in acid |
 |
| The cotton shown here is being used as a support only. The linen cut
outs are to be dipped in the battery acid (see shallow dish with skull and
crossbones). |
 |
| Here's the O of H2SO4 in the acid, with attached cable tie |
 |
| Laying out the formula on the cotton support after dipping in acid. |
 |
| Formula complete. Now all we have to do is wait. |
 | |||
| Look more closely, and one sees there is a change. for a start the treated linen is now a light beige colour, while the untreated control (removed centre of the O) is the original white. |
 |
| Here one can see clearly the beige colour of the acid-treated linen against the original linen shirt. |
 |
| Here's a supplementary test that was most revealing. The "2" of H2SO4 has been placed on a hot radiator (which will be wiped with bicarbonate later to neutralise acid contamination). |
 |
| The "2" quickly darkens still further at the higher temperature. Note the difference between it and untreated linen. |
 |
| When the heated "2" was replaced, something unexpected happened. When trying to position it correctly, it fell apart! |
 |
| Here's the same against the same original black background. Gentle prodding with a pencil showed that all the linen was now highly fragile, while still relatively "white" (something of a surprise). |
 |
| Here's the fragmented "O" with untreated control. The colour change is subtle, but there's no doubting that the acid has seriously degraded the linen while not greatly altering its appearance. |
Why should that be? Why should linen be chemically attacked by H2SO4, of concentration intermediate between battery acid and conc.H2SO4 (after evaporation of some water) but not cotton?
The original purpose of the experiment was to test the hypothesis that sepia Shroud image might have been formed from dye mordants (alum, iron sulphates) releasing sulphuric acid into the fibres of the linen which on evaporation of water produced local high concentrations of H2SO4 leading to discoloration of the cloth. That hypothesis was NOT confirmed in these experiments with actual high strength H2SO4. Instead there was a totally unexpected finding that linen differs from cotton in being highly susceptible in some way to acid, losing its tensile strength,and indeed disintegrating under light tension. Why? Might that finding have something to say about the nature of the TS image?
Hypothesis
Firstly: can the difference between linen and cotton be rationalized. Yes. I believe it can. Cotton is well known as the purest source of cellulose in nature (the figure generally quoted is 90%). Cellulose is celebrated for its resistance to chemical attack (being made use of in chemical laboratories for filter paper able to withstand a wide range of harsh reagents). Cotton contains virtually no hemicelluloses, which despite their name have virtually nothing in common with cellulose. Hemicelluloses are chemically far more reactive than cellulose.
Now let's consider linen, or the bast fibres from the stem of flax plants from which it is derived. Linen is generally quoted as having 5-15% of polysaccharides that are NOT cellulose, comprising mainly hemicelluloses and pectins.
Late addition: a literature source found by googling (linen hemicellulose) that confirms the major difference regarding linen and cotton re the major difference re hemicellulose content.
(References to detailed carbohydrate compositions have proved elusive, but if I find any more, I'll add them here).
Like: this passage from Caspar von Uffhofen in 'The Mystery of the Shroud"
Linen yarn is quoted above as having 11-17% hemicellulose, and a mere 1.8% pectin. How strange that the hemicellulose of linen is rarely if ever mentioned in Shroud image literature, especially as cellulose is chemically so inert by comparison. Some sources, naming no names, discuss linen as though it were 100% cellulose. I have even seen the hemicellulose referred to as "an impurity"
A general description of flax fibres is that they comprise a cellulose-hemicellulose cross-linked combination embedded in a gel matrix of pectins (though some perhaps most of the pectin is lost when flax fibres are retted to make linen).
The presence of hemicelluloses could explain why linen is greatly weakened by sulphuric acid, whereas cotton is not. What's more the acid must affect the entire fibre to account for mechanical weakening - not just the most superficial part of the fibre. Those reading this who are familiar with the literature on the Turin Shroud image will no doubt be aware of where this is all leading.
Here's an image I showed in a posting over 2 years ago on my specialist Shroud site:
In fact, i returned to the same theme again and again a year later. See this one in particualr entitled: Why are Shroud image fibres mechanically weaker than non-image bearing fibres? Pyrolysis of core hemicelluloses?
My proposal that core hemicelluloses might have a role to play in explaining the weakness of Shroud image fibres sadly failed to attract attention elsewhere. Maybe it didn't fit with the prevailing narrative that the Shroud image and associated chemical modification is entirely confined to the most superficial part of the linen fibre, typically quoted as 200nm thick, and that absence of colour in the core of the fibre means there has been no physical or chemical change there (despite the mechanical weakness of TS image fibres discovered by Raymond N.Rogers in his 'sticky tape' sampling of TS fibres, relative to non-image fibres). Or maybe it was the tying of the core susceptibility idea to the scorch hypothesis that guaranteed that even if seen and commented upon, it would be quickly dismissed and forgotten.
Takeaway message? Too much emphasis has been placed on visual coloration in image fibres, as if chemical changes to flax or linen carbohydrates always produce a yellow colour. That's led to the paradigm that the Shroud image is incredibly superficial and so, ipso facto, the totality of physical and chemical changes must also be restricted entirely to the most superficial part of the fibre, corresponding roughly with the primary cell wall. One simply cannot make that assumption, given that chemical change, sufficient to weaken fibres, need not be accompanied by a handy self-indicating colour change. Example from this posting: there was only a slight darkening of linen fibres when exposed to sulphuric acid despite massive weakening of fibre tensile strength, but only in linen - not cotton. So who's to say that the process that produced the image on the TS was not - as is generally maintained - one that was too subtle to be explained by conventional physics or chemistry, and was instead one that degraded linen fibres, notably the core hemicelluloses, producing major changes at the chemical and mechanical level, but ones that are not obvious under the microscope?
There is every reason to put the uv excimer lasers away, at least for now, and consider all the various ways in which a medieval artisan might have set about imprinting an image onto linen, one that he and his masters could claim was that left on Joseph of Arimathea's linen.
Update: here's a comment I posted earlier to shroudstory.com, essentially addressing the point raised here - namely changes to SCW on image capture that are maybe not as visible as those to the superficial PCW:
April 1, 2015 at 4:30 am
Forgive my saying, Piero, but you are making the same error as
Paolo Di Lazzaro and his ENEA colleagues in assuming that because the TS
image COLOUR is superficial, confined (maybe) to the PCW or a Rogers’
type coating, then ipso facto there are no changes to less superficial parts of the fibre.
Are you not forgetting something? Image fibres are weaker than non-image fibres. That was shown by the ease with which they could break off and stick to Rogers’ Mylar adhesive tape. It would take more than changes in the PCW – an ultra- thin skin on a much thicker mainly SCW fibre – to render the entire fibre brittle and prone to fracture. (Let’s not forget that the Mylar initially stripped off entire fibres – not image “ghosts” – the latter not being seen/inferred until image fibres were subsequently pulled away from the tape).
Try by all means to get the Vatican to consider your AFM scanning. But let’s dispense with the notion that the imaging process affected only the most superficial parts of the fibre. The core of a linen fibre is not entirely crystalline cellulose. There are significant amounts of hemicelluloses and pectins there too – the so-called non-cellulosic polysaccharides (NCPs).Their physical and/or chemical disruption could compromise the strength and integrity of the entire fibre. Being in a minority relative to cellulose microfibrils means that changes to those NCPs might not be so readily visible as coloration, which is where we came in.
Are you not forgetting something? Image fibres are weaker than non-image fibres. That was shown by the ease with which they could break off and stick to Rogers’ Mylar adhesive tape. It would take more than changes in the PCW – an ultra- thin skin on a much thicker mainly SCW fibre – to render the entire fibre brittle and prone to fracture. (Let’s not forget that the Mylar initially stripped off entire fibres – not image “ghosts” – the latter not being seen/inferred until image fibres were subsequently pulled away from the tape).
Try by all means to get the Vatican to consider your AFM scanning. But let’s dispense with the notion that the imaging process affected only the most superficial parts of the fibre. The core of a linen fibre is not entirely crystalline cellulose. There are significant amounts of hemicelluloses and pectins there too – the so-called non-cellulosic polysaccharides (NCPs).Their physical and/or chemical disruption could compromise the strength and integrity of the entire fibre. Being in a minority relative to cellulose microfibrils means that changes to those NCPs might not be so readily visible as coloration, which is where we came in.
Update: Thursday 2nd April
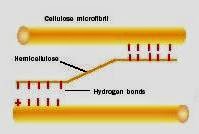
Here's a schematic diagram found by internet search that shows the inferred relationship between cellulose microfibrils and hemicellulose crosslinking 'bracing'. (It's part of a discussion of expandable primary cell walls but there's every reason to suppose that it applies equally well to the secondary cell wall, despite the PCW and SCW fulfilling different roles at different points in the plant cell's life history).
Note that the bonding between cellulose and hemicellulose is not via permanent covalent chemical bonds. It's via hydrogen bonds, the weaker nature of which (individually) can be more than compensated by their numbers. The crucial difference where susceptibility to thermal and chemical treatments are concerned is that it's relatively easy to break hydrogen bonds which may or may not subsequently return to their original geometry. So we have here a possible mechanism by which image imprinting could seriously impair the tensile strength of linen image fibres without producing colour, and without affecting cellulose crystallinity, the latter inferred by Rogers from unaltered birefringence pattern under crossed-polaroids,. There's an analogy that can be made with double-stranded DNA, the two strands of which are held together in the double helix via hydrogen-bonding between base pairs. Once can separate the stands by heating and "melting". However, to get them to return to their original correctly base-paired configuration one has to "anneal", ie. cool very slowly, which allows the separated strands to 'experiment' with different arrangement until the optimum most-stable pattern is restored.
Conclusion: imprinting linen with a superficial image using one or other technology may not result in visible change to the inner core, but that does not preclude serious disruption occurring at the molecular level that may not be visible either to the unaided eye or under the microscope. In short: superficiality of visible coloration does not necessarily mean that the input energy was of an exceptionally subliminal kind (e.g. high energy/short pulse) unknown to science. Maybe Shroud image fibres are more seriously compromised than their faint yellow colour would indicate. Their mechanical weakness is the clue to more profound physical or chemical disruption having occurred within the SCW cores of the fibres. Maybe medieval technology was capable of producing that particular presentation known to paramedics of minor superficial change concealing more serious 'internal injury'.
Further update: have just this minute googled (shroud turin image fibres weaker):
Am I the only one to consider that altered mechanical strength to be an important pointer to change at the molecular level (as important, if not more so, as change in visible colour, reflectance spectrum, uv fluorescence, dichroism under crossed polaroids etc etc)? Yup, the first 6 listings above are all to my own postings (but then I have been muttering about fragile SCW hemicelluloses for well over two years now).
Technical footnote regarding different grades of sulphuric acid
The so-called "concentrated sulphuric acid" is purchased as a dense oily liquid, which is 98% H2SO4, 2% H2O. It is highly dangerous, and not the sort of chemical one wants around the house or even garage. It's to do with its avidity (old-fashioned chemical term) for water. One gets a hint if one dilutes it with water following the safe and correct technique, which is add the con acid in a thin stream to water with constant stirring. The temperature rises quickly (one may see steam) and one may hear a rumbling sound (from cavitating steam bubbles). Never ever imagine one can dilute by adding water to conc H2SO4. Try doing that and the whole lot is liable to come back in your eyes and face in short order: hot sulphuric acid is not to be recommended. Why the projectile event? Because of density differences the water initially sits on top of the denser H2SO4. There's then the violent heat of dilution at the interface that then causes greater contact, more heat, more contact etc etc, and steam propulsion out the beaker does the rest. This blogger was once a few yards from a lab technician who did it the wrong way (I blame the researcher who failed to enquire ahead of time as to the level of her chemical -handling credentials). Fortunately no permanent injuries resulted, but there was a lot of unpleasant clean up and neutralisation (sodium bicarbonate) to be done of splattered acid - over bench surfaces, reagent bottles, lab notes and lab coats.
I shall return shortly to discuss what happens when conc. sulphuric acid is allowed to stand (in a safe place) exposed to moist air, and compare with the experiment described above when pre-diluted acid of battery strength (approx. 35%) is similarly exposed.
Back again. Yes, it's not hard to find references to the volume increase that is seen when conc.H2SO4 is exposed to air, and neither is it difficult to find references to solutions of dilute(d) sulphuric acid becoming more concentrated as they evaporate and shrink in volume. What I have not been able to find thus far is quantitative data. One would like to know the final concentration of sulphuric acid after conc.H2SO4 has picked up the maximum amount of water vapour, and conversely the final concentration of H2SO4 after the dilute acid has evaporated to its maximum concentration of H2SO4. One assumes the two acids - conc. and dilute- converge to the same figure for a given atmospheric moisture content. It's obviously somewhere between 35% and 98%, but I have no information on whether it's nearer 50% or 90%. However- one things for certain: the concentration never reaches 98% for physical reasons, due to the aforementioned hygroscopic nature of 98% H2SO4, and never acquires the ability to cause rapid charring of fabric, as seen when it is treated with conc.(98%) H2So4, recalling the findings above of at best a faint beige color on standing for 2 days in air (with non-evaporated liquid still visible).
 |
| Reminder from a previous posting here on what can happen when fabric is treated with conc.(98%) H2SO4. |
Final addition: what about the initial hypothesis - namely that traces of sulphuric acid from mordants may have caramelized the linen to produce the TS image we see today(maybe as a ghost). Well, there was a very slight colouring of the linen exposed to H2SO4 over 2 days, more beige than yellow or sepia, but hardly enough one would think to account for the TS image, faint though it is. If a high concentration of H2SO4 on its own cannot produce a reasonably visible sepia coloration, then it's unlikely that trace amounts can do so, unless one invokes catalysis from other constituents (Occam's Razor!).
Never mind. The H2SO4 hypothesis has led me to consider another chemical scenario that is not too far removed from H2SO4, and one moreover that would seem to tick a lot of boxes, even the tricky ones like ultra-superficiality/confinement of image to the PCW, half-tone effect, bleaching with diimide, even Adler and Heller's "blood-first" conclusion that has always been an Achilles heel for a medieval provenance.
So what's the new idea? Be patient, folks. It will be the subject of my next posting, arriving in the next few days.
Nope, this is the final addition, added Friday 24th April, some 3 weeks and 5 postings after the one above. It's my response to the ludicrous accusation (same old, same old) from the Usual Suspect on Dan Porter's shroudstory site that I have "recycled" his ideas. The crucial word is "fumigation". No sooner had I hit on the idea that HNO3 vapour (as distinct from liquid solution) might be less problematical as a chemical developing agent than I quickly adopted the term "fumigation". What are the one-word alternatives? I can't think of any off the top of my head, and fumigation is easy to understand. But the usual suspect has also used that term, albeit in an entirely different pro-authenticity context for post-mortem imaging in a 1st century tomb that was carried out, he claims, according to a complex routine (I shall spare readers what for me is eye-glazing detail), and in any case has never been put his model to the experimental test, the relying he says on "thought experiments".
Anyway, just to let folk know that I was doing some house-keeping in my work area today, and came across this note that I wrote to myself, almost certainly in the first few days of April when I realized I had reached the end of the road with sulphuric acid, and was wondering where to go from there:
Here it is verbatim, faulty punctuation, crossings-out an' all, but no deployment of the f word at this stage.
"Time for a total rethink on the TS image. Maybe there
























2 comments:
prof prem raj pushpakaran writes -- John Brown Francis Herreshoff, the great metallurgical chemist who invented process for making Sulphuric Acid was born on February 7th 1850!!!
This is such nice and useful information for us. I really appreciate this blog to have this kind of knowledge and it is good to know the latest updates. Also explore Sulphuric Acid Suppliers
Post a Comment